Benzoyl Peroxide
Bernardino D. Madsen, MT (ASCP) - Instructor
- Medical Laboratory Technology Program
- Casper College
- School of Health Science
- Casper, Wyoming
Quality 20 gr benzoylThe stomach 125 Both the pancreas and the duodenum are well protected within the superior retroperitoneum deep inside the stomach skin care giant crossword buy 20gr benzoyl mastercard. Therefore acne 2 weeks pregnant discount benzoyl 20gr otc, in order to skin care bandung buy benzoyl 20gr free shipping maintain an harm to either one of them skin care 20s discount benzoyl 20 gr fast delivery, there should be other related injuries. It requires a excessive degree of suspicion and important clinical acumen as well as aggressive radiographic imaging to determine an damage to these organs this early in the presentation. As a end result, the surgeon is incessantly confronted with the dilemma of choosing between a number of preoperative investigations and tons of surgical procedures. These accidents happen at the junction of free (intraperitoneal) components of the duodenum with fixed (retroperitoneal) components. A high index of suspicion based on the mechanism of injury and physical examination findings may lead to additional diagnostic research. After several hours, the duodenal contents extravasate in to the peritoneal cavity, with the development of peritonitis. Because of the retroperitoneal location of the duodenum, and its shut proximity to numerous different viscera and main vascular buildings, isolated penetrating injuries of the duodenum are uncommon. The need for abdominal exploration is normally dictated by related injuries, and the prognosis of duodenal harm is normally made in the working room. These usually occur when crushing the duodenum between the backbone and a steering wheel or handlebar, or when some other force is applied to the duodenum. These accidents can be related to flexion/distraction fractures of the L1�L2 vertebrae � the Chance fracture. Less frequent in deceleration damage patterns are Theoretically, duodenal perforations are associated with a leak of amylase and different digestive enzymes, and it has been suggested that determination of the serum amylase concentration may be helpful within the diagnosis of blunt duodenal damage. Although serial determinations of serum amylase are higher than a single, isolated willpower on admission, sensitivity continues to be poor, and needed delays are inherent in serial determinations. If the serum amylase level is elevated on admission, a diligent search for duodenal rupture is warranted. Plain X-rays of the stomach are useful when fuel bubbles are current within the retroperitoneum adjoining to the proper psoas muscle, around the best kidney or anterior to the higher lumbar spine. They also can present free intraperitoneal air, and, though not often seen, air within the biliary tree additionally has been described. An upper gastrointestinal series using water-soluble contrast materials can present optimistic leads to 50 per cent of patients with duodenal perforations. Meglumine (gastrografin) ought to be infused by way of the nasogastric tube after which swallowed, and the study ought to be accomplished under fluoroscopic control with the patient in the right lateral position. If no leak is observed, the investigation continues with the patient in the supine and left lateral positions. If the Gastrografin research is unfavorable, it ought to be followed by administration of barium to allow the detection of small perforations extra readily. It could be very delicate to the presence of small amounts of retroperitoneal air, blood or extravasated distinction from the injured duodenum, particularly in youngsters. The presence of periduodenal wall thickening or haematoma with out extravasation of contrast material should be investigated with a gastrointestinal examine with Gastrografin. In truth, because of its the stomach 127 and their remedy should embrace duodenal diversion. The management of all full-thickness duodenal lacerations ought to embody enough external periduodenal drainage. Timing of the operation is also essential as mortality rises from 11 per cent to forty per cent if the time interval between harm and operation is more than 24 hours. Alternatively, the fourth a part of the duodenum may be mobilized by dividing the ligament of Treitz and gently dissecting with proper index finger within the avascular airplane behind the transverse duodenum. Combining this with the Kocher manoeuvre permits the index fingers to be introduced together from both sides and thereby to exclude a posterior perforation of the transverse a part of the duodenum. It requires distinct manoeuvres to diagnose damage (cholangiogram and direct visible inspection), and complicated methods to repair them. The first and second parts of the duodenum are densely adherent and dependent for their blood supply on the head of the pancreas; subsequently, the analysis this can be a uncommon harm of the duodenum particular to patients with blunt trauma. This is because of the gradual increase of the scale of a haematoma because the breakdown of the haemoglobin makes it hyperosmotic, with resultant fluid shifts in to it. One choice is to open the serosa, evacuate the haematoma with out violation of the mucosa, and thoroughly repair the wall of the bowel. The concern is that this may convert a partial tear to a full-thickness tear of the duodenal wall. Another option is to rigorously explore the duodenum to exclude a perforation, leaving the intramural haematoma intact and planning nasogastric decompression postoperatively. These accidents are related to associated pancreatic harm, blunt or missile harm, involvement of more than seventy five per cent of the duodenal wall, damage of the first or second a part of the duodenum, a time interval of more than 24 hours between damage and repair, and related common bile duct damage. In these high-risk injuries, a quantity of adjunctive operative procedures have been proposed in an try to reduce the incidence of dehiscence of the duodenal suture line. The closure should be oriented transversely, if potential, to keep away from luminal compromise. Longitudinal duodenotomies can usually be closed transversely if the length of the duodenal harm is less than 50 per cent of the circumference of the duodenum. If primary closure would compromise the lumen of the duodenum, several options have been beneficial. A pedicled mucosal graft, as a technique of closing giant duodenal defects, has been instructed, utilizing a section of jejunum or a gastric island flap from the physique of the abdomen. Although encouraging in experimental research, the medical software of each methods has been limited, and suture line leaks have been reported. When such harm occurs distal to the ampulla of Vater, closure of the distal duodenum and roux-en-Y duodenojejunal anastomosis is acceptable. A direct anastomosis to a roux-en-Y loop sutured over the duodenal defect in an end-to-side fashion is the procedure of selection. External drainage should be offered in all duodenal injuries because it affords early detection and management of the duodenal fistula. The drain is preferably a simple, soft silicone rubber, closed system positioned adjacent to the restore. In order to protect the duodenal repair, the gastrointestinal contents � with their proteolytic enzymes � can be diverted, a practice that would additionally make the administration of a possible duodenal fistula easier. This is incessantly the case with accidents of the first, third or fourth a half of the duodenum, the place mobilization is technically not troublesome. It was first described in 1954 as a way of administration of a precarious closure of the duodenal stump after a gastrectomy. The initial beneficial reports on the efficacy of this technique to lower the incidence of dehiscence of the duodenorrhaphy have, however, not been supported by more recent reports. The fashioning of a feeding jejunostomy at the preliminary laparotomy in sufferers with duodenal harm and in depth belly trauma (Abdominal Trauma Index rating >25) is highly beneficial. After primary repair of the duodenum, a gastrotomy is made at the antrum alongside the greater curvature.
Buy 20 gr benzoyl free shippingSymptoms embrace vomiting skin care 35 purchase 20gr benzoyl fast delivery, headache and visible disturbance acne prescription medication cheap 20 gr benzoyl mastercard, generally accompanied by belly distension skin care house philippines buy benzoyl 20gr with visa. Delayed cases might present with intermittent/ uncommon fever acne treatment home remedies cheap benzoyl 20 gr overnight delivery, rash arthralgia, anaemia and muscle aches. Epidural catheter-associated an infection Pathogenesis Associated with epidural injections. Clinical options Early presentation could additionally be delicate and clinical diagnosis difficult to set up. Treatment Shunt removing and therapy with antimicrobials based on causative pathogen. Antibiotic therapy to eradicate current infections, which Prevention Aseptic methods on insertion and antimicrobial prophylaxis; antimicrobial shunts are also being utilized in some centres. Complications of infective endocarditis embody embolic phenomena, which may be fatal when involving the cerebrovascular system. Risk components for growing infective endocarditis embrace congenital and bought structural cardiac defects and intracardiac prostheses. Pathogenesis the characteristic manifestation of infective endocarditis is the vegetation. A vegetation types when micro organism from the blood colonise microthrombi current on broken endothelium on the valve surface. Endothelial damage happens as a result of abnormal intracardiac jets and the presence of prosthetic material. Right-sided infective Aetiology Infective endocarditis is often attributable to Gram-positive microorganisms. Staphylococci are the most common causative microorganisms (both Staphylococcus aureus and coagulase unfavorable Medical Microbiology and Infection Lecture Notes, Fifth Edition. This is commonly brought on by prior antibiotic administration or when an infection is as a outcome of of fastidious microorganisms. Transthoracic and transoesophageal echocardiography visualise vegetations and evaluate valvular integrity. As the variety of bacteria circulating in the blood is small, you will want to inoculate the maximum permitted quantity of blood in to each tradition bottle to optimise the yield. Serological assays have to be used to diagnose infective endocarditis caused by Coxiella burnettii, Brucella spp. The diagnosis of infective endocarditis is established utilizing the Duke standards, which is based primarily on culture results and presence of a vegetation. Vegetations could also be seen on echocardiography, adherent to the intracardiac portion of pacing leads. Epidemiology Intracardiac gadget infection occurs in 1�2% of implanted gadgets per yr. Pathogenesis It is probably going that system related infections are attributable to micro organism being introduced during implantation. Management Synergistic bactericidal antibiotics are needed for extended intervals of time (4�6 weeks). Surgical debridement and valvular surgical procedure could additionally be required when response to treatment is poor or valvular destruction is intensive. Clinical features Clinical features vary from non-specific signs corresponding to fever, malaise and night time sweats to those related to infective endocarditis. Aetiology Device related infections are mostly caused by coagulase negative staphylococci and S. Prevention Previously antibiotics have been really helpful for all these considered to be vulnerable to infective endocarditis previous to bacteraemia producing procedures and operations. Currently, most tips suggest antibiotic prophylaxis for much less than those Laboratory analysis Frequently, blood cultures are adverse as are wound swabs, normally due to prior antibiotic use. Aetiology Myocarditis is often viral in origin, though toxic myocarditis occurs in diphtheria and continual myocarditis is a function of trypanosomiasis and chlamydial an infection. Laboratory analysis A viral aetiology can solely be proved if virus is detected in altered myocardium by way of endocardial biopsy or at necropsy. Viral: nucleic acid detection or isolation from pharyngeal washings, faeces or pericardial fluid (see Chapter 16); Bacterial: Culture of pericardial fluid and blood; Echocardiography could show a brilliant pericardium and the presence of pericardial fluid. Management Acute therapy is with analgesia with nonsteroidal anti-inflammatory medicine. Clinical or echocardiographic options of cardiac tamponade require emergency pericardiocentesis. Antifungal and antibacterial medication ought to be given, relying on the causative microorganism. Aetiology Staphylococcus aureus is the most common reason for osteomyelitis, however different microorganisms may be necessary in certain patients groups or conditions. In acute ostemyelitis, signs are normally current for days or a couple of weeks, however continual osteomyelitis often refers to instances the place an infection has endured for months or years in spite of antimicrobial therapy (and sometimes surgery); lifeless bone is often present. Pathogenesis and epidemiology Osteomyelitis can happen in all kinds of patient groups and any bone may be affected, but a relatively small variety of pathogens are involved. Osteomyelitis can present acutely or develop slowly over a interval of weeks to months, or even years. If the affected bone is near the skin there could also be swelling, ache, erythema and tenderness over the affected space. In infants and young children, decreased use of an affected limb is a crucial signal. The duration of signs usually varies with the virulence or progress characteristics of the causative microorganism. Discharging sinuses from the infected bone to the skin might happen in continual Staphylococcus aureus osteomyelitis. Fungi Benzylpenicillin Amoxicillin or benzylpenicillin benzylpenicillin, metronidazole Amoxicillin Cefotaxime Ceftazidime According to susceptibilities. Surgery, to allow drainage of pus and dbridement of lifeless bone, may be necessary, e notably in persistent osteomyelitis. Antibiotic treatment is dependent upon the causative microorganism, highlighting the need for appropriate microbiological investigation. Radiologically-guided or operative bone samples for culture are the diagnostic procedures of choice. Routine culture, mycobacterial tradition and fungal culture of operative or biopsy samples is critical. Infection might affect native (natural) joints or prosthetic joints and these are discussed separately because of differences in pathogenesis, aetiology and management. Synovial tissue is extremely vascular and lacks a basement membrane � this may facilitate passage of bacteria from the blood in to a native joint.
Purchase benzoyl 20 gr onlineThus acne medication prescription buy generic benzoyl 20 gr online, the energy H of the response is H = a hundred thirty + 57 - eighty - 156 = -49 kcal Because 1 cal = 4 skin care over 40 discount 20 gr benzoyl fast delivery. This approach might find some use in estimating the power related to chemical instability skin care not tested on animals order benzoyl 20 gr line. Additional Applications of Thermochemistry Thermochemical knowledge are necessary in lots of chemical calculations skin care procter and gamble generic benzoyl 20gr online. Heat-of-mixing information can be used to determine whether or not a reaction similar to precipitation is going on in the course of the mixing of two salt options. If no response takes place when dilute solutions of the salts are blended, the heat of response is zero. In the neutralization of a weak electrolyte by a robust the Efficiency of a Heat Engine An essential consideration is that of the potential for changing warmth in to work. Not only is warmth isothermally unavailable for work, it could by no means be transformed utterly in to work. Falling water could be made to do work owing to the distinction within the potential vitality at two completely different levels, and electric work could be done because of the distinction in electrical potential (emf). A heat engine (such as a steam engine) likewise can do useful work by utilizing two heat reservoirs, a "source" and a "sink," at two totally different temperatures. Only part of the warmth on the source is converted in to work, with the rest being returned to the sink (which, in practical operations, is commonly the surroundings) at the decrease temperature. The fraction of the warmth, Q, at the supply transformed in to work, W, is called the effectivity of the engine: W Efficiency Q (3�33) sixty three If Thot = Tcold in equation (3�36), the cycle is isothermal and the efficiency is zero, confirming the sooner assertion that heat is isothermally unavailable for conversion in to work. Imagine a hypothetical steam engine operating reversibly between an higher temperature Thot and a lower temperature Tcold. It absorbs heat Qhot from the recent boiler or source, and via the working substance, steam, it converts the quantity W in to work and returns warmth Qcold to the cold reservoir or sink. It is defined as Q rev T and for an infinitesimal change, S= dH = H T dT P (3�39) It is known that heat circulate in the operation of the engine follows the temperature gradient, so that the warmth absorbed and rejected could be related on to temperatures. Lord Kelvin used the ratios of the two heat portions Qhot and Qcold of the Carnot cycle to set up the Kelvin temperature scale: Q sizzling Thot = Q cold Tcold (3�35) (3�40) By combining equations (3�33) by way of (3�35), we are ready to describe the efficiency by Efficiency = Q sizzling - Q cold Thot - Tcold = Q hot Thot (3�36) It is noticed from equation (3�36) that the higher Thot becomes and the lower Tcold becomes, the larger is the efficiency of the engine. When Tcold reaches absolute zero on the Kelvin scale, the reversible warmth engine converts warmth completely in to work, and its theoretical effectivity becomes unity. Because absolute zero is considered unattainable, however, an efficiency of 1 is impossible, and heat can by no means be fully transformed to work. This statement may be written utilizing the notation of limits as follows: W =1 (3�37) lim Tcold zero Q Thus, the term Qhot /Thot is called the entropy change of the reversible course of at Thot, and Qcold /Tcold is the entropy change of the reversible process at Tcold. The entropy change Shot in the course of the absorption of warmth from Thot is positive, nonetheless, as a result of Qhot is constructive. At the decrease temperature, Qcold is negative and the entropy change Scold is adverse. It could be seen that solely part of the warmth Qhot (1000 cal) is transformed to work (200 cal) within the engine. To this, add the entropy change undergone by the environment in order to acquire the total entropy change. The heat capability of water is 18 cal/deg and that of ice is 9 cal/deg inside this temperature range. The reversible change of water at -10 C to ice at -10 C is carried out as follows: Tfinal = zero. In an irreversible course of, the entropy change of the entire system or universe (a system and its surroundings) is all the time constructive as a end result of Ssurr is always less than Ssyst in an irreversible course of. In mathematical symbols, we write, Suniv = Ssyst + Ssurr > zero (3�43) H2 O(s,0) H2 O(s,-10); H2 O(l,-10) H2 O(s,-10); and this will function a criterion of spontaneity of a real process. Equations (3�42) and (3�43) summarize all the possibilities for the entropy; for irreversible processes (real transformations) entropy all the time increases till it reaches its maximum at equilibrium, at which point it remains invariable. Two examples of entropy calculations will now be given, first, a reversible process, and second, an irreversible process. In this reversible isothermal process, the heat of vaporization Hv required to convert the liquid to the vapor state is 10,500 cal/mole. For the entropy change of the surroundings, the water must be thought of to be in equilibrium with a big tub at -10 C, and the warmth liberated when the water freezes is absorbed by the tub and not utilizing a vital temperature enhance. The entropy is obtained by calculating the entropy adjustments for a number of reversible steps. Entropy and Disorder the second regulation offers a criterion for deciding whether a course of follows the natural or spontaneous direction. Thermodynamic systems described by macroscopic properties similar to T, P, or composition can be described by means of microscopic portions corresponding to molecular random motions. The impossibility of converting all thermal energy in to work results from the "disorderliness" of the molecules present within the system. Disorder can be seen as the number of ways the inside of a system may be arranged in order that from the surface the system appears the identical. Unlike the thermodynamic definition of entropy in equation (3�39) or (3�40), to perceive what a configuration means. The initial state corresponds to V1 and the ultimate state corresponds to V2, which is larger than V1: V1 = 1 2 V2. Now / think about two independent particles; the probability of discovering both in V1 after V2 is available is P = (1 2)2 = 1 4. Once the system has expanded, it is extremely inconceivable that they are going to be discovered by chance solely in V1. The larger the variety of configurations, the extra disordered a system is considered. Thus, the increase in entropy with rising number of configurations is properly described by Boltzmann concept given in equation (3�46). In different phrases, a pure good crystal has only one attainable configuration, and based on equation (3�46), its entropy is zero [i. Each of those terms may be evaluated independently; specifically, the primary integral is calculated numerically by plotting C P /T versus T. These criteria, along with the entropy criterion of equilibrium and spontaneity, are listed in Table 3�3. The difference in sign between G and Suniverse implies that the condition for a process being spontaneous has modified from an increase of the whole entropy, Suniverse > zero, to a lower in Gibbs free power, G < 0. However, Gibbs free energy is simply a composite that expresses the whole change in entropy by method of the properties of the system alone. Criteria of Equilibrium and Spontaneity When net work can not be obtained from a course of, G is at a minimum, and G = zero. On the opposite hand, from equation (3�62) a adverse free vitality change written as G < 0 signifies that the method is a spontaneous one.
Discount benzoyl 20gr free shippingInfections are all the time symptomatic acne hat discount benzoyl 20 gr without prescription, starting abruptly with fever acne and birth control discount benzoyl 20 gr otc, headache acne 4 weeks pregnant purchase benzoyl 20 gr with visa, myalgia skin care questionnaire order benzoyl 20 gr without prescription, arthralgia, conjunctivitis, sore throat, abdominal pain, nausea and vomiting. IgG antibodies may mirror previous publicity; Nucleic acid amplification tests on blood; Virus isolation only in specialised laboratories. Oral ribavarin may be used for prophylaxis in shut contacts with needlestick harm. Ebola and Marburg haemorrhagic fever Virus Members of the Filoviridae household of viruses. Crimean�Congo haemorrhagic fever Virus Member of the Bunyaviridae household of viruses. Humans become infected through tick bites or contact with blood, tissue or excreta of infected animals. The virus is distributed over Eastern Europe, (particularly within the former Soviet Union) around the Mediterranean, in northwestern China, Africa, the Middle East and the Indian subcontinent. Person-to-person unfold is often confined to healthcare employees taking care of severe cases. Complication Bleeding tendency from venepuncture sites, epistaxis, haematuria, haematemesis and melaena from day 4 of illness onwards. The antiviral drug ribavirin is energetic in vitro and has proven some benefit significantly if given inside 5 days of onset. Sudden onset of fever, severe headache, sore throat, nausea and vomiting, basic fatigue and malaise. Flushed Part four Self-assessment Self-assessment questions Some questions might have more than one correct reply. A Most micro organism are surrounded by a rigid cell envelope (cell wall) containing peptidoglycan. A Lipopolysaccharide is a element of the cell envelope of Gram-positive bacteria. Streptococcus pneumoniae) the capsular material varies in composition and can be utilized in serotyping to distinguish between different types of the same species of microorganism. E Fimbriae are skinny, hair-like buildings produced on the surface of some micro organism, enabling them to adhere to host tissues. A All micro organism could be cultured in laboratory media, however certain microorganisms require specific nutrients to be provided. Bacteria that develop only in the presence of oxygen are called obligate aerobes, these rising only within the absence of oxygen are called obligate anaerobes, and people growing within the presence or absence of oxygen are referred to as facultative anaerobes. Mannitol salt agar plates are an example of selective growth media used for the recovery and development of staphylococci from scientific samples. Chocolate agar, containing lysed blood, is an enriched progress medium used for tradition of some bacteria which have particular progress necessities provided by the blood. A Endocarditis B Urinary tract infection C Osteomyelitis D Skin infection E Respiratory tract an infection 3. A the use of certain antibiotics is a risk issue for development of an infection with this microorganism. Peptostreptococcus species are obligate aerobes and may trigger abscesses at many anatomical sites. B G Tropheryma whipplei is an intracellular pathogen, which causes illness more prevalent in females. A Meningococcus B Gonococcus C Pneumococcus D Moraxella catarrhalis E Veillonella species 7. A Ten % of people are asymptomatic carriers of the microorganism within the nasopharynx. A Escherichia only B Enterobacter solely C Klebsiella solely D Escherichia, Enterobacter and Klebsiella E Salmonella, Shigella, Serratia, Proteus and Yersinia E F 288 Self-assessment questions 8. A Haemophlius influenzae B Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans) C Cardiobacterium hominis D Eikenella corrodens E Klebsiella pneumoniae 9. Chapter 10 Pseudomonas, Legionella and other environmental Gram-negative bacilli 10. B C Self-assessment questions 289 D E Unlike different pseudomonads, Acinetobacter spp. E Non-cholera Vibrio species may also cause diarrhoea and occasionally trigger cellulitis. C Few spirochaetes could be cultured and prognosis is normally made by serological exams. E Spirochaetes, like Treponema pallidum, could be visualised in scientific specimens by darkish subject microscopy. A the non-specific antibody check detects antibody to lipids (cardiolipin) released from the microorganism throughout early infection. C They are the commonest explanation for acute bacterial enterocolitis, and an infection is commonly brought on by the handling of raw chickens or consumption of the undercooked meat. A Helicobacter pylori is the most typical cause of duodenal ulceration and gastric most cancers. B Infection with Helicobacter pylori occurs generally in early childhood and normally becomes chronic, usually life-long. C the prevalence of infection with Helicobacter pylori is low in international locations with poor sanitation. E Treatment of an infection usually entails a proton pump inhibitor to scale back gastric acidity coupled with one antimicrobial. A Vibrio cholerae 01 is the cause of cholera, which is characterised by profuse watery diarrhoea. Vibrio cholerae varieties characteristic pink colonies on thiosulphate-citrate-bile salt-sucrose agar. Treatment of cholera is with rehydration and often ciprofloxacin to shorten the period of illness. A Bacteroides B Prevotella D 290 Self-assessment questions C Porphyromonas D Fusobacterium E Leptotrichia 13. C Chlamydia trachomatis is the most typical explanation for sexually transmitted infections in the developed world. D Infection with Chlamydia trachomatis is commonly treated with doxycycline or azithromycin. E Chlamydophila psittaci can additionally be a standard explanation for sexually transmitted an infection. Viral an infection is all the time identified by direct detection of all or a half of the virus. C Viral nucleic acids may be single- or double-stranded, round or linear, and have negative or positive polarity. D A stay attenuated virus vaccine is available for the prevention of infection with the Measles virus. E Infection with the Mumps virus is generally characterised by swollen parotid salivary glands.
Purchase 20 gr benzoyl with mastercardPauling1 suggested the chance of hybridization to account for the quadrivalence skin care during pregnancy discount 20 gr benzoyl. It is supposed simply to separate out the varied types of complexes which are discussed in the literature acne 6 year old buy generic benzoyl 20gr on-line. A extremely systematized classification of electron donor�acceptor interactions is given by R acne 5 year old buy benzoyl 20gr with visa. The dsp2 or sq. planar construction is predicted to be the complicated formed because it makes use of the lower-energy 3d orbital acne and diet benzoyl 20 gr lowest price. By the preparation and examine of numerous complexes, Werner deduced many years in the past that this is indeed the structure of the advanced. The digital configuration of the metallic ion resulting in filled 3d levels is these are directed towards the corners of a tetrahedron, and the construction is named an sp3 hybrid because it entails one s and three p orbitals. In a double bond, the carbon atom is taken into account to be sp2 hybridized, and the bonds are directed toward the corners of a triangle. The transition parts, such as iron, copper, nickel, cobalt, and zinc, seem to make use of their 3d, 4s, and 4p orbitals in forming hybrids. These hybrids account for the differing geometries typically discovered for the complexes of the transition metallic ions. Table 10�2 exhibits some compounds by which the central atom or steel ion is hybridized in a different way and the geometry that outcomes. A useful but not inviolate rule to observe in estimating the kind of hybridization in a metal ion complex is to select that complex in which the metal ion has its 3d levels crammed or that can use the lower-energy 3d and 4s orbitals primarily within the hybridization. For instance, the ground-state electronic configuration of Ni2+ could be given as and thus the d2 sp3 or octahedral construction is predicted because the construction of this advanced. Chelates (see following section) of octahedral construction could be resolved in to optical isomers, and in an elegant examine, Werner2 used this technique to show that cobalt complexes are octahedral. The presence of unpaired electrons in a metal ion complex can be detected by electron spin resonance spectroscopy. As a consequence of this isomerism, solely cis-coordinated ligands-ligands adjoining on a molecule-will be readily replaced by reaction with a chelating agent. Vitamin B12 and the hemoproteins are incapable of reacting with chelating agents because their metal is already coordinated in such a way that only the transcoordination positions of the steel can be found for complexation. In distinction, the steel ion in certain enzymes, such as alcohol dehydrogenase, which accommodates as a result of no stabilization is gained over that which the d2 sp3 configuration already possesses. A calorimetric method for assaying procainamide in injectable solutions relies on the formation of a 1:1 advanced of procainamide with cupric ion at pH 4 to four. The distinction between complexation and the formation of natural compounds has been proven by Clapp. Chlorophyll and hemoglobin, two extraordinarily important compounds, are naturally occurring chelates concerned within the life processes of vegetation and animals. Albumin is the principle provider of varied steel ions and small molecules in the blood serum. This reality partly explains why people are much less susceptible to copper poisoning than are dogs. The binding of copper to serum albumin is essential because this steel is probably involved in a quantity of pathologic conditions. In the process of sequestration, the chelating agent and metal ion type a water-soluble compound. Ethylenediaminetetraacetic acid is widely used to sequester and take away calcium ions from onerous water. On the opposite hand, these two compounds react at an elevated temperature to yield a salt, the constituent molecules of that are held together by major valence bonds: the dotted line in the complicated of equation (10�1) indicates that the 2 molecules are held collectively by a weak secondary valence drive. It is not to be thought of as a clearly defined bond however somewhat as an overall attraction between the 2 aromatic molecules. Some of the bonds in a chelate may be ionic or of the first covalent kind, whereas others are coordinate covalent hyperlinks. When the ligand provides one group for attachment to the central ion, the chelate known as monodentate. Molecules with two and three donor teams are referred to as bidentate and tridentate, respectively. Ethylenediaminetetraacetic acid has six points for attachment to the steel ion and is accordingly hexadentate; nonetheless, in some complexes, solely four or five of the groups are coordinated. Resonance in a donor�acceptor complicated of trinitrobenzene (acceptor, top) and hexamethylbenzene (donor, bottom). The vitality of attraction between the constituents might be less than 5 kcal/mole for many natural complexes. Instead, one molecule polarizes the opposite, leading to a type of ionic interplay or cost switch, and these molecular complexes are sometimes referred to as charge transfer complexes. For instance, the polar nitro teams of trinitrobenzene induce a dipole within the readily polarizable benzene molecule, and the electrostatic interplay that outcomes leads to advanced formation: X-ray diffraction studies of complexes fashioned between trinitrobenzene and aniline derivatives have proven that one of the nitro teams of trinitrobenzene lies over the benzene ring of the aniline molecule, the intermolecular distance between � the 2 molecules being about 3. This outcome strongly suggests that the interplay includes bonding between the electrons of the benzene ring and the electron-accepting nitro group. A issue of some significance within the formation of molecular complexes is the steric requirement. Hydrogen bonding and different effects should also be thought-about, and these are mentioned in connection with the precise complexes considered on the next pages. The distinction between a donor�acceptor and a charge transfer advanced is that in the latter type, resonance makes the primary contribution to complexation, whereas in the former, London dispersion forces and dipole�dipole interactions contribute extra to the soundness of the complicated. On the left facet of the figure, weak dispersion and dipolar forces contribute to the interplay of A and D; on the best aspect of the determine, the interplay of A and D results from a big transfer of cost, making the electron acceptor trinitrobenzene negatively charged (A-) and leaving the donor, hexamethylbenzene, positively charged (D+). If, as within the case of hexamethylbenzene�trinitrobenzene, the resonance is pretty weak, having an intermolecular binding power G of about � 4700 energy, the complicated is referred to as a donor� acceptor complex. If, on the opposite hand, resonance between the charge switch structure (D+ A-) and the uncharged species (D A) contributes greatly to the binding of the donor and acceptor molecule, the advanced is called a cost switch advanced. Finally, these complexes sure together by van der Waals forces, dipole�dipole interactions, and hydrogen bonding however lacking charge switch are identified merely as molecular complexes. Iodine types 1:1 charge switch complexes with the drugs disulfiram, chlomethiazole, and tolnaftate. These medicine have acknowledged pharmacologic actions of their own: 202 Disulfiram is used towards alcohol habit, clomethiazole is a sedative�hypnotic and anticonvulsant, and tolnaftate is an antifungal agent. Each of those medicine possesses a nitrogen� carbon�sulfur moiety (see the accompanying construction of tolnaftate), and a complex might end result from the transfer of charge from the pair of free electrons on the nitrogen and/or sulfur atoms of these drugs to the antibonding orbital of the iodine atom. They attributed the interaction between caffeine and a drug such as a sulfonamide or a barbiturate to a dipole�dipole pressure or hydrogen bonding between the polarized carbonyl teams of caffeine and the hydrogen atom of the acid. A secondary interplay most likely happens between the nonpolar components of the molecules, and the resultant complicated is "squeezed out" of the aqueous section owing to the good inner strain of water.
Buy benzoyl 20 gr on-lineImmunisation towards a variety of potential ailments; including Haemophilus influenzae acne young living buy benzoyl 20 gr visa, pertussis (whooping cough) acne vs rosacea 20 gr benzoyl with amex, mumps acne location meaning purchase benzoyl 20 gr with amex, diphtheria acne hoodie 20gr benzoyl otc, polio, Streptococcus pneumoniae (pneumococcus), Neisseria meningitidis and Clostridium tetani (tetanus). Infections not current at the time of admission to a hospital may be either derived in the community (community-acquired infections) or acquired throughout hospitalisation (hospital-acquired infections). Prevention includes good aseptic method and the appropriate use of antimicrobial prophylaxis for certain procedures. Firstly, cross infection from different patients; these are prevented by interrupting the route of transmission. These want particular preventative and control strategies, in addition to approaches outlined above. Examples of healthcareassociated an infection Examples of sources and microorganisms associated with totally different healthcare-associated infections are given in Table 24. Urinary tract infection This, in many surveys, is the most typical nosocomial an infection, and is usually a complication of urethral catheterisation or other urinary tract operative procedures. The supply of the infecting microorganism may be endogenous or exogenous (being introduced throughout urological procedures or manipulation of catheter drainage systems). Prevention consists of the usage of sterile instruments, closed-drainage urinary catheter methods, and careful aseptic technique throughout urinary catheterisation and subsequent catheter care. Surgical site infection the incidence of surgical site (wound) an infection varies in accordance with the sort of surgery (Table 24. Streptococcus pyogenes: much less widespread than staphylococcal infections, but might end in rapid native spread (cellulitis or fasciitis) and bloodstream infection. Exogenous acquisition could occur intraoperatively or post-operatively, both by the airborne route or by direct contact (crossinfection). Preoperative measures: decontamination of skin surfaces pre-operatively with antiseptics; prophylactic antimicrobials for operations with a big threat of wound an infection. The proper dose of the proper antimicrobial(s) need to be given at the proper time pre-operatively Lower respiratory tract infection this necessary explanation for morbidity and mortality in hospitalised sufferers is often a complication of intubation (anaesthesia, intensive care), which by-passes the normal physical defenses of the respiratory tract. Gowns, aprons and masks must be worn by all these entering the room; palms must be washed earlier than and after coming into. Isolation procedures Hospitals require a coverage for isolating patients with particular transmissible infections (source isolation) and for isolating sure immunocompromised patients. For enteric infections, procedures are required for secure disposal of faeces and urine. Some hospitals have specific infectious illnesses wards or isolation wards for such patients. A legal code of apply is in place, which ensures this and the Care Quality Commission inspects healthcare institutions towards this code. Methods embody patients carrying infection reporting playing cards that they give to healthcare staff. Provides recommendation on all matters relating to the prevention and spread of an infection. Major outbreaks It is essential that hospitals, as well as the group, have in place systems for managing main outbreaks. If an outbreak occurs, there are several stages in the investigation, including collection of epidemiological information, screening of hospital instances and setting up an acceptable outbreak management management plan. Most outbreaks of healthcare-associated infection embrace colonised and infected sufferers. In addition, occupational well being should be represented on this committee, to be sure that the chance of infection to healthcare employees is minimised. There are numerous methods of surveillance, including laboratory-based surveillance. England is uncommon in also setting and reviewing targets for reductions in infections included in a few of the mandatory surveillance actions. Lengths of hospital stay at the second are so brief for some circumstances (including day surgical cases) that surveillance Table 24. Biological warfare Plans need to be in place, both in hospitals and the group, in case of contamination with biological material, including smallpox and anthrax. Precautions include acceptable protecting clothes, prophylaxis and immunisation. Prevention of an infection to healthcare employees Healthcare staff can shield themselves from acquiring infections by several mechanisms. Immunisation: against varied infections together with hepatitis B; Barrier protection: healthcare employees ought to perform a risk assessment when coping with any patient, and may assume that a patient could additionally be potentially infectious and apply universal precautions. These are precautions that must be taken for every patient contact to stop the unfold of infection. Avoidance of needlestick accidents is essential within the unfold of blood-borne viruses; Strict isolation: that is required for highly infectious or harmful pathogens. Lassa fever, these patients need to be located in designated high-risk isolation hospitals. The spectrum of severity ranges from self-limiting viral infections that resolve without medical consultation to life threatening systemic bacterial illness or acute airway compromise. Above the cords, the mucosal surfaces are colonised by bacteria, beneath the cords is generally maintained freed from microorganisms by body defences. Infections of the higher respiratory tract therefore embrace these affecting the nose, pharynx, tonsils, sinuses, larynx and ears. A number of systemic viral infections may present with related higher respiratory tract signs. Re-infections are frequent because of antigenic variety inside each of the viral teams. Viral infections the frequent cold syndrome Definition An acute, self-limiting syndrome comprising coryza (clear nasal discharge), accompanied by other upper respiratory tract signs and indicators. Peak incidence in temperate areas is in the winter, whereas in the tropics, incidence peaks in the course of the wet season. The predominant symptom is coryza, which is commonly accompanied by cough, sneezing and Medical Microbiology and Infection Lecture Notes, Fifth Edition. Secondary bacterial an infection may lead to bacterial sinusitis or otitis media (see below). Pharyngitis and conjunctivitis may occur, with enterovirus or adenovirus an infection. Bacterial infections Streptococcus pyogenes and infrequently different b-haemolytic streptococci (groups C and G). Arcanobacterium haemolyticum, Neisseria gonorhoeae) can uncommonly cause pharyngitis. These results may be used to instigate remedy (immunocompromised patients) or to inform an infection control procedures.
Discount benzoyl 20 gr on-lineAlthough still used with medication skin care obagi purchase benzoyl 20 gr on-line, these halogenated hydrocarbons are not used in cosmetic aerosols and have been replaced by nitrogen and unsubstituted hydrocarbons skin care manufacturers quality benzoyl 20gr. Since propellants represent the vast majority (>99%) of the dose delivered by a metered dose inhaler acne keloidalis generic benzoyl 20 gr otc, it should be nontoxic to the affected person acne 2 week cheap 20gr benzoyl. Many examples of resolution pairs are known, nonetheless, during which the "cohesive" attraction of A for A exceeds the "adhesive" attraction existing between A and B. Similarly, the engaging forces between A and B may be higher than those between A and A or B and B. In the case of liquid pairs that present negative deviation from the law, nonetheless, the addition of B to A tends to cut back the vapor stress of A to a higher extent than may be accounted for by the straightforward dilution impact. Chloroform and acetone manifest such an attraction for each other through the formation of a hydrogen bond, thus further reducing the escaping tendency of each constituent. Reactions between dipolar molecules, or between a dipolar and a nonpolar molecule, may lead to negative deviations. The interplay in these circumstances, nevertheless, is often so weak that no definite compound could be isolated. Accordingly, the dissimilarity of polarities or inside pressures of the constituents ends in a higher escaping tendency of both the A and the B molecules. The whole vapor pressure usually reveals a maximum at one particular composition if the deviation is sufficiently large. Liquid pairs that demonstrate optimistic deviation are benzene and ethyl alcohol, carbon disulfide and acetone, and chloroform and ethyl alcohol. It describes the habits of both part of a real liquid pair only when that substance is current in high focus and thus is considered to be the solvent. The molecules of solute, being in comparatively small number within the two areas of the diagram, are completely surrounded by molecules of solvent and so reside in a uniform surroundings. With these mixtures, distillation produces both pure A or pure B plus a mixture of fixed composition and constant boiling point. This latter is called an azeotrope (Greek: "boil unchanged") or azeotropic combination. The composition of this combination is correct and reproducible sufficient that the answer can be used as a standard in analytic chemistry. Mixtures of water and acetic acid and of chloroform and acetone yield azeotropic mixtures with maxima in their boiling level curves and minima in their vapor pressure curves. Mixtures of ethanol and water and of methanol and benzene each show the reverse conduct, specifically, minima in the boiling level curves and maxima in the vapor stress curves. Boiling begins, and distillation may be effected when the sum of the partial pressures of the two immiscible liquids simply exceeds the atmospheric pressure. This precept is utilized in steam distillation, whereby many natural compounds insoluble in water can be purified at a temperature nicely below the purpose at which decomposition occurs. Bromobenzene can thus be distilled at a temperature sixty one C under its regular boiling point. Steam distillation is especially useful for obtaining risky oils from plant tissues without decomposing the oils. Distillation of Binary Mixtures the connection between vapor stress (and therefore boiling point) and composition of binary liquid phases is the underlying precept in distillation. The larger the vapor stress of a liquid-that is, the extra unstable it is-the lower is the boiling point. Because the vapor of a binary mixture is at all times richer within the extra risky constituent, the method of distillation can be used to separate the more volatile from the less risky constituent. A combination of those substances having the composition a is distilled on the boiling level b. The vapor rising within the column is met by the condensed vapor or downward-flowing liquid. As the rising vapor is cooled by contact with the liquid, a number of the lower-boiling fraction condenses, and the vapor accommodates more of the risky part than it did when it left the retort. Therefore, because the vapor proceeds up the fractionating column, it turns into progressively richer within the more unstable element B, and the liquid returning to the distilling retort becomes richer in the much less volatile element A. These are referred to as colligative properties (Greek: "collected together") because they depend mainly on the number quite than on the character of the constituents. Because the solute under dialogue right here is considered to be nonvolatile, the vapor pressure of the solvent, p1, is equivalent to the total pressure of the solution, p. It is extra convenient to express the vapor stress of the answer when it comes to the concentration of the solute somewhat than the mole fraction of the solvent, and this can be accomplished in the following method. The sum of the mole fractions of the constituents in a solution is unity: X1 + X2 = 1 Therefore, X1 = 1 - X2 (5�12) (5�11) where X1 is the mole fraction of the solvent and X2 is the mole fraction of the solute. The outcome may additionally be acknowledged as a percentage; the vapor stress of the answer has been lowered zero. The mole fraction, n2 /(n1 + n2), is sort of equal to , and could also be changed by, the mole ratio n2 /n1 in a dilute answer corresponding to this one. Then, the relative vapor strain lowering could be expressed in phrases of molal focus of the solute by setting the burden of solvent w1 equal to a thousand g. Theoretical plot of the traditional boiling point for water (solvent) as a perform of molality in solutions containing sucrose (a nonvolatile solute) in increasing concentrations. Note that the normal boiling point of water will increase as the focus of sucrose increases. The boiling level of an answer of a nonvolatile solute is greater than that of the pure solvent owing to the fact that the solute lowers the vapor strain of the solvent. The vapor strain curve for the answer lies under that of the pure solvent, and the temperature of the answer have to be elevated to a worth above that of the solvent to have the ability to attain the normal boiling point. In dilute solutions, X2 is equal roughly to m/(1000/M1) [equation (5�16)], and equation (5�19) could be written as k M1 m (5�20) Tb = one thousand or Tb = K b m (5�21) Moreover, as a result of p is a constant, the boiling level elevation could also be thought of proportional to p/p, the relative where Tb is named the boiling level elevation and Kb is known as the molal elevation constant or the ebullioscopic constant. Stated one other method, Kb is the ratio of the boiling point elevation to the molal focus in an especially dilute resolution by which the system is approximately best. The previous dialogue constitutes a believable argument leading to the equation for boiling point elevation. A more satisfactory derivation of equation (5�21), however, entails the application of the Clapeyron equation, which is written as Tb Vv - V1 = Tb (5�22) p Hv the place Vv and V1 are the molar quantity of the gasoline and the molar volume of the liquid, respectively, Tb is the boiling level of the solvent, and Hv is the molar warmth of vaporization. Boiling point elevation of the solvent as a end result of addition of a solute (not to scale). If a solute is dissolved in the liquid on the triple level, the escaping tendency or vapor stress of the liquid solvent is lowered below that of the pure solid solvent. The temperature should drop to reestablish equilibrium between the liquid and the strong. Because of this fact, the freezing point of an answer is at all times decrease than that of the pure solvent.
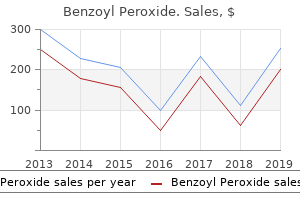
Benzoyl Peroxide: 20 gr
Proven 20 gr benzoylIf the method could be performed infinitely slowly in order that no work is expended in supplying kinetic vitality to the piston korean skin care effective benzoyl 20 gr, and if the piston is considered to be frictionless so that no work is finished in opposition to the pressure of friction acne toner purchase benzoyl 20 gr with amex, all the work is used to broaden or compress the vapor acne studios scarf discount 20 gr benzoyl. One of one of the best examples of reversibility is that involved in the measurement of the potential of an electrochemical cell utilizing the potentiometric technique skin care doctors orono buy generic benzoyl 20 gr on line. In similar fashion, it could be deduced that the minimal work in a reversible compression of the system will also lead to equation (3�8), because at every stage Pex is simply infinitesimally larger than P. No work is achieved if a perfect gas expands freely in to a vacuum, the place P = zero, as a result of any work completed is decided by the exterior pressure. Of course, if the external pressure is regularly increased, the gas is compressed quite than expanded, and work is done on the system quite than by the system in an isothermal reversible course of. When the opposite is true, V2 < V1, and ln(V2 /V1) is unfavorable due to gasoline compression, work is completed by the system, in order that its energy increases Equation (3�10) provides the utmost work done in the expansion as well as the warmth absorbed, as a result of Q = E - W, and, as might be proven later, E is the same as zero for a perfect fuel in an isothermal course of. The conditions of this drawback are just like these of Example 3�2, except that equation (3�10) can now be used to obtain the The enhance in enthalpy, H, is equal to the warmth absorbed at constant strain by the system. It is the warmth required to enhance the internal power and to perform the work of expansion, as seen by substituting H in equation (3�19), Q P = H2 - H1 = and writing equation (3�18) as H= E+P V (3�21) H (3�20) the place the subscript V signifies that quantity is fixed. The ratio between these quantities defines the molar warmth capacity at constant quantity: Cv dqv = dT E T (3�14) V Ideal Gases and the First Law An best fuel has no inside stress, and hence no work wants be carried out to separate the molecules from their cohesive forces when the fuel expands. Therefore, d w = 0, l and the primary legislation turns into dE = d q l (3�15) the heat absorbed in a response carried out at atmospheric pressure is independent of the number of steps and the mechanism of the response. When electrical work, work against surfaces, or centrifugal forces are thought-about, one must write as H = Q P - Wnonatm (3�23) Thus, the work accomplished by the system within the isothermal enlargement of a perfect fuel is equal to the warmth absorbed by the gas. These thermodynamic properties are, of course, associated by the definition of H, equation (3�21). Heat of Formation For any reaction represented by the chemical equation aA + bB = cC + dD the enthalpy change could be written as H = H products - H reactants (3�28) (3�29) Summary Some of the particular restrictions which have been placed on the first legislation as a lot as this level in the chapter, together with the resultant modifications of the regulation, are introduced collectively in Table 3�1. A comparison of the entries in Table 3�1 with the material that has gone earlier than will function a comprehensive evaluate of the primary legislation. Under this situation, the heat exchanged in the course of the course of equals the change in enthalpy based on equation (3�20), Q P = H. Thus, the change in enthalpy accompanying a chemical response remains a function only of temperature as acknowledged in equation (3�27). It can be possible that a response takes place in a closed container; in such a case the heat exchanged equals the change in inner power. Thermochemistry deals with the warmth modifications accompanying isothermal chemical reactions at constant pressure or the place H = enthalpy per mole (called the molar enthalpy) and a, b, c, and d are stoichiometric coefficients. It is thought that solely the molar enthalpies of compounds, both as reactants or merchandise, contribute to the change of enthalpy of a chemical response. Additional symbols, (l) for liquid and (aq) for dilute aqueous answer, will be present in subsequent thermochemical equations. However, as early as 1840, Hess confirmed that as a result of H depends solely on the initial and last states of a system, thermochemical equations for several steps in a reaction could be added and subtracted to obtain the warmth of the overall reaction. The commonplace warmth of formation of gaseous carbon dioxide is H (25 C) = -94,052 cal. The state of matter or allotropic form of the weather also have to be specified in defining the usual state. Equation (3�30) states that when 1 mole of stable carbon (graphite) reacts with 1 mole of gaseous oxygen to produce 1 mole of gaseous carbon dioxide at 25 C, ninety four,052 cal is liberated. This signifies that the reactants comprise ninety four,052 cal in excess of the product, in order that this quantity of warmth is evolved in the course of the reaction. Heats of Reaction from Bond Energies In Chapter 2, energies of bonding had been discussed when it comes to binding forces that maintain molecules (intramolecular bonding) or states of matter (noncovalent forces) collectively. For instance, the energies associated with covalent bonding between atoms vary from 50 to 200 kcal/mole. Electrovalent or ionic bonds occurring between atoms with opposing permanent expenses could be a lot stronger than a covalent bond. These forces vary in strength due to interaction between attraction and repulsion. Therefore, the online bonding vitality that holds a collection of atoms together in a molecule is the additive results of all the individual bonding energies. In a chemical response, bonds may be broken and new bonds could additionally be formed to give rise to the product. The web vitality associated with the reaction, the warmth of response, can be estimated from the bond energies which would possibly be broken and the bond energies which would possibly be shaped through the response process. Many of the frequent covalent and other bonding-type energies may be commonly present in books on thermodynamics, like the ones listed in the footnote in the opening of this chapter. Because some heat is absorbed in the ionization of the weak electrolyte, the warmth advanced falls beneath the worth for the neutralization of completely ionized species. Thus, knowledge of H of neutralization allows one to differentiate between sturdy and weak electrolytes. Another essential software of thermochemistry is the willpower of the number of calories obtained from numerous meals. The topic is mentioned in biochemistry texts as properly as books on food and diet. Similarly, gases expand naturally from greater to lower pressures, and solute molecules diffuse from a area of upper to one of decrease focus. Maximum work is obtained by conducting a spontaneous course of reversibly; however, the frictional losses and the necessity of finishing up the method at an infinitely gradual rate preclude the potential for full reversibility in actual processes. The second legislation refers to the probability of the incidence of a course of based mostly on the noticed tendency of a system to strategy a state of energy equilibrium. The historical growth of the thermodynamic property that explains the natural tendency of the processes to happen, now called entropy, has its origins in research of the efficiency of steam engines, from which the following statement was made: "A steam engine can do work solely with a fall in temperature and a circulate of heat to the decrease temperature. Chemical reactions are normally carried out at fixed temperature and fixed strain. Thus, equations involving G are of particular curiosity to the chemist and the pharmacist. It was once thought that at constant strain a adverse H (evolution of heat) was itself proof of a spontaneous reaction. Many natural reactions do occur with an evolution of heat; the spontaneous melting of ice at 10 C, nonetheless, is accompanied by absorption of warmth, and numerous different examples can be cited to prove the error of this assumption. The purpose H is usually regarded as a criterion of spontaneity could be seen from the familiar expression (3�67). Hence, equation (3�64) can be written as Difference in bond energies or G = enticing energies between merchandise and reactants, H Change in probability - in the course of the process, (3�69) T S One can state that G will turn out to be unfavorable and the response will be spontaneous either when the enthalpy decreases or the likelihood of the system increases on the temperature of the reaction. Hence, the entropy increases, and S = 6 cal/mole deg is sufficiently constructive to make G unfavorable, despite the optimistic worth of H.
References - McDuffie RW Jr, Litin RB, Blundon KE: Urethrovesical suspension (Marshall- Marchetti-Krantz). Experience with 204 cases, Am J Surg 141(2):297n298, 1981.
- Amlie RN, Borgeois B, Huxtable R: Priapism in preterm infant, Urology 9:558n559, 1977.
- Shen J, Overland M, Sinclair A, et al: Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra, Differentiation 92(4):169n182, 2016.
- Bayer, I., Kyzer, S., Chaimoff, C. A new approach to primary strengthening of colostomy with Marlex mesh to prevent paracolostomy hernia. Surg Gynecol Obstet. 1986; 163:579-580.
- Morrissey JJ, Klahr S: Differential effects of ACE and AT1 receptor inhibition on chemoattractant and adhesion molecule synthesis, Am J Physiol 274(3 Pt 2):F580nF586, 1998.
|


