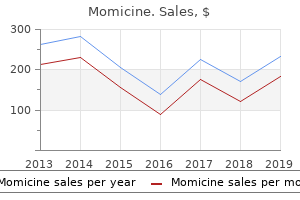
Momicine
Mitra Taghizadeh, MS, MT(ASCP) - Former Assistant Professor
- Department of Medical and Research Technology
- University of Maryland School of Medicine
- Baltimore, Maryland
Cheap momicine 500mg lineWhether these agents also induce folic acid deficiency in the fetus is much less certain infection from pedicure buy momicine 100mg with visa, because the fetus seems to be efficient in drawing on available maternal stores of folic acid antibiotics without insurance order momicine 250 mg fast delivery. Low maternal folate levels antibiotics for acne online trusted momicine 100 mg, however antibiotic resistance reasons generic momicine 250mg visa, have been proposed as a mechanism for the increased incidence of defects observed in infants uncovered in utero to some anticonvulsants. In a 1984 article, investigators reported research on the connection among folic acid, anticonvulsants, and fetal defects (74). In the retrospective part of this research, a group of 24 girls who were treated with phenytoin and other anticonvulsants produced 66 infants, of whom 10 (15%) had major anomalies. A second group of 22 epileptic women was then given supplements of daily folic acid, 2. This group produced 33 newborns (32 pregnancies, one set of twins) with no defects, a big difference from the group not receiving supplementation. Negative associations between anticonvulsant-induced folate deficiency and start defects have also been reported (70,75). Investigators studied a gaggle of epileptic girls taking anticonvulsants and observed only two defects (2. Another group of researchers observed 20 infants (15%) with defects from 133 ladies taking anticonvulsants (75). Folate levels have been normally within the regular vary (normal thought of to be serum >1. Whole embryo cultures of rats have been examined with valproic acid and folinic acid, a folic acid derivative (76). Teratogenic doses of valproic acid brought on a big reduction in embryonic ranges of formylated tetrahydrofolates and increased the degrees of tetrahydrofolate by inhibition of the enzyme glutamate formyltransferase. The results of this inhibition would have serious penalties on embryonic development, together with neural tube closure (77). A review of teratogenic mechanisms involving folic acid and antiepileptic remedy was printed in 1992 (78). Animal research cited indicated that valproic acid disrupts folic acid metabolism, possibly by inhibiting key enzymes, quite than by reducing concentrations of the vitamin, whereas phenytoin may act on folic acid by both mechanisms (78). The reviewers concluded that folic acid supplementation could also be effective in preventing some poor being pregnant outcomes in epileptic ladies. In a 2013 casecontrol research from Japan masking the period 20012012, 360 girls who had given birth to spina bifida-affected offspring have been compared with 2333 girls who had given delivery to offspring with out spina bifida (80). In 2000, the Japanese government recommended that girls planning a being pregnant should take 0. Because the prevalence rate of spina bifida, 56 per 10,000 whole births, had not changed in 11 years, the authors beneficial that necessary meals fortification with folic acid must be carried out (80). Placental Abruption Several articles have proposed that maternal folic acid status is associated with placental abruption (25,26,28,66,81). In a evaluation and evaluation of 506 consecutive circumstances of abruptio placentae, defective folate metabolism was discovered as a predisposing factor in 97. The authors theorized that folic acid deficiency early in being pregnant brought on irreversible damage to the fetus, chorion, and decidua, resulting in abruption, abortion, premature supply, low start weight, and fetal malformations. Other research have discovered that 60% of their patients with abruption had been folate poor, however their numbers were too small for statistical evaluation (82). In different series, no correlation was found between low levels of folic acid and this complication (16,69,83). A second group of investigators studied folate ranges in one hundred and one preeclamptic and 17 eclamptic women and compared them with 52 regular controls and 29 ladies with overt megaloblastic anemia (84). Abortions and Placenta Previa Several papers have related folic acid deficiency with abortion (25,26,66,78,eighty one,8587). The explanation for some abortions, as proposed by some, is faulty folate metabolism in early pregnancy, producing irreversible injury to the fetus and placenta (81). In a collection of sixty six patients with early spontaneous abortions, the incidence of folate deficiency was the same as in those with uncomplicated pregnancies (88). These researchers did discover a relationship between low folic acid ranges and placenta previa. However, others found no proof of an association between folate deficiency and either abortion or antepartum hemorrhage (12). Premature Birth and Low Birth Weight the relationship amongst prematurity, low delivery weight, and folic acid levels has been investigated. In one research, significantly decrease folate ranges had been measured within the blood of low-birth-weight neonates than in that of normal-weight infants (18). The incidences of both untimely supply and infants with start weight <2500 g have been elevated in folate-deficient mothers in a 1960 report (22). These sufferers all had severe megaloblastic anemia and a poor standard of vitamin. In a later study of 510 infants from folate-deficient moms, 276 (54%) weighed 2500 g compared with a predicted incidence of eight. A study of women with uterine bleeding throughout pregnancy discovered a significant association between serum folate and low birth weight (86). Similarly, another examine reported a major relationship between folate levels on the finish of the 2nd trimester and newborn birth weight (89). A 1992 report described the effects of supplementation with ferrous sulfate (325 mg/day) and folic acid (1 mg/day), starting on the first prenatal go to, on infant birth weight (90). In distinction, others have found no affiliation between folic acid deficiency and prematurity (27,69,ninety one,92) or between serum folate and start weight (12,69,93,94). Two reviews have alluded to issues with high folic acid ranges in the mother during being pregnant (95,96). An isolated case report described an anencephalic fetus whose mother was underneath psychiatric care (95). She had been handled with very high doses of folic acid and nutritional vitamins B1, B6, and C. A 1984 examine examined the impact of folic acid, zinc, and other vitamins on pregnancy consequence (96). Total complications of being pregnant (infection, bleeding, fetal distress, prematurity or death, pregnancy-induced hypertension, and tissue fragility) had been related to high serum folate and low serum zinc levels. The rationalization provided for these stunning findings was that folate inhibits intestinal absorption of zinc, which, they proposed, was responsible for the problems. An increased danger of adverse fetal consequence can be lowered by folic acid supplementation in a minimum of two teams of women. A specific dosage recommendation has not been positioned for ladies receiving anticonvulsants, but four or 5 mg/day has been used. Levels of folic acid are relatively low in colostrum however as lactation proceeds, concentrations of the vitamin rise (101103). Folate ranges in newborns and breastfed infants are consistently larger than those in mothers and normal adults (104,105).
Buy momicine 500 mg otcUtilizing varied concentrations of the drug in a whole-embryo tradition system and direct administration to pregnant females (200 mg/kg subcutaneously every four hours for 3 doses) during organogenesis antibiotics ok during pregnancy discount 250 mg momicine overnight delivery, in vitro vidarabine showed the best potential to interfere with embryonic improvement antibiotics for acne cons purchase momicine 250mg with amex, whereas in vivo acyclovir had the very best teratogenic potential antibiotics not safe during pregnancy momicine 500 mg mastercard. In this study virus malware removal buy 250mg momicine with mastercard, the in vitro reproductive toxicity of ddA was lower than that of the other brokers, except for zidovudine. The in vivo toxicity was lower than that of acyclovir, vidarabine, and ganciclovir, and equal to that observed with zalcitabine and zidovudine. A 1994 report in contrast this toxicity amongst zidovudine and three newer compounds, didanosine, stavudine, and zalcitabine (4). Whereas vital inhibition of blastocyst formation occurred with a 1 µmol/L concentration of zidovudine, stavudine, and zalcitabine toxicity was not detected until a hundred µmol/L, and no toxicity was noticed with didanosine as a lot as one hundred µmol/L. An earlier examine found no cytotoxicity in preimplantation mouse embryos uncovered to didanosine concentrations 500 µmol/L (2). A 1995 report described the impact of exposure to a relatively high concentration of didanosine (20 µmol/L vs. In pregnant macaques, didanosine was administered by fixed infusion at a dose of either 42. The compound crossed the placenta by simple diffusion, resulting in fetal:maternal concentration ratios for both doses of roughly 0. Using a perfused time period human placenta, investigators concluded in a 1992 publication that the placental switch of didanosine was most probably because of passive diffusion (9). Two other studies, once more utilizing perfused human placenta, discovered that only about 50% of the drug would be passively transferred to the fetal circulation (10), and at roughly half the speed of zidovudine (11). In distinction to zidovudine (see Zidovudine), no metabolite was detected within the placenta (10). Drug concentrations in the maternal blood, fetal blood, and amniotic fluid at slightly more than 1 hour after the dose have been 295, 42, and <5 ng/mL, respectively, within the first patient, and 629, 121, and a hundred thirty five ng/mL, respectively, in the second woman. Although single-drug-level determinations are difficult to interpret, a research printed in 1993 discovered that human placental first-pass metabolism was not the explanation for these low fetal and amniotic fluid ranges (13). For every drug, the expected fetal:maternal plasma drug focus ratios at steady state with each of the three fashions had been close to these actually observed in pregnant macaques. This examine also described the pharmacokinetics of oral didanosine (60 mg/m2) in infants at day 1 and at week 6 after birth. The Antiretroviral Pregnancy Registry reported, for January 1989 through July 2009, potential data (reported before the outcomes have been known) involving 4702 stay births that had been uncovered through the 1st trimester to one or more antiretroviral brokers (16). There were 627 outcomes exposed to didanosine (370 within the 1st trimester and 257 within the 2nd/3rd trimesters) in combination with other antiretroviral agents. There were 22 delivery defects (17 in the 1st trimester and 5 within the 2nd/3rd trimesters). In reviewing the delivery defects of prospective and retrospective (pregnancies reported after the outcomes had been known) registered circumstances, the Registry concluded that, apart from isolated instances of neural tube defects with efavirenz exposure in retrospective reports, there was no other pattern of anomalies (isolated or syndromic) (16). At term, a pale, male infant was delivered, who developed respiratory misery shortly after start. The infant acquired a transfusion and was apparently doing properly at 10 weeks of age. Because no different reason for the anemia could possibly be discovered, the authors attributed the condition to bone morrow suppression, most likely to zidovudine (17). She was receiving trimethoprim/sulfamethoxazole, didanosine, stavudine, nevirapine, and vitamin B supplements (specific vitamins and dosage not given) that had been began before conception. Defects observed at post-mortem included ventriculomegaly, an Arnold Chiari malformation, sacral spina bifida, and a lumbosacral meningomyelocele. Two critiques, one in 1996 and the other in 1997, concluded that every one girls presently receiving antiretroviral remedy ought to proceed to obtain remedy throughout being pregnant and that treatment of the mother with monotherapy must be thought-about insufficient remedy (20,21). The similar conclusion was reached in a 2003 evaluate with the added admonishment that therapy should be steady to stop emergence of resistant viral strains (22). The molecular weight (about 236) is low enough that excretion into milk ought to be expected. Placental switch and fetal disposition of 23-dideoxycytidine and 23-dideoxyinosine in the rhesus monkey. The transfer of anti-human immunodeficiency virus nucleoside compounds by the term human placenta. In vitro models to predict the in vivo mechanism, price, and extent of placental transfer of dideoxynucleoside drugs towards human immunodeficiency virus. A retrospective survey of 1232 sufferers exposed to diethylpropion during pregnancy discovered no difference within the incidence of defects (0. No impairment of fertility, teratogenicity, or fetotoxicity was noticed in animal research with doses up to 9 occasions these utilized in people (3,4). The use of an appetite suppressant (diethylpropion hydrochloride) throughout being pregnant. This use has resulted, nonetheless, in important issues of the reproductive system in both female and male offspring (112). More than 400 cases of clear cell adenocarcinoma have been reported to the Registry. The first-known case of adenosquamous carcinoma of the cervix in an exposed affected person was described in 1983 (15). In a second case, a fatal malignant teratoma of the ovary developed in a 12-year-old uncovered lady (16). The incidence of cervical or vaginal structural changes has been reported to occur in up to 85% of uncovered ladies, though most studies place the incidence in the 22%58% vary (2,three,5,7,12,2124). Selection bias was eliminated by analyzing solely those sufferers recognized by record evaluation. Patients referred by physicians and self-referrals had a lot larger charges of defects, about 49% and 43%, respectively. Almost all of the defects had been confined to the cervicalvaginal fornix area, with solely 14 patients having vaginal changes unique of the fornix and nearly all of these being incomplete transverse septums (10). Alterations within the body of the uterus have led to concern relating to elevated being pregnant wastage and untimely births (8,22,2831). Increased rates of spontaneous abortions, untimely births, and ectopic pregnancies are well established by these latter reviews, although the relationship to the abnormal modifications of the cervix and/or vagina is still unclear (8). Serial observations of vaginal epithelial changes indicate that the frequency of such changes decreases with age (4,17,24). One group of investigators found that although anomalies within the upper genital tract elevated the chance for poor being pregnant outcome, they could not relate particular modifications to specific types of outcomes (34). The diploma of hirsutism was age related, with the mean ages of severely and mildly hirsute ladies being 28. Abnormalities thought to occur at higher frequencies include the following: Epididymal cysts Hypotrophic testis Microphallus Varicocele Capsular induration Altered semen (decreased rely, concentration, motility, and morphology) An improve in problems with passing urine and urogenital tract infections has additionally been noticed (40). In addition, a examine of 828 uncovered males discovered no increase over controls for danger of genitourinary abnormalities, infertility, or testicular cancer (47).
Purchase 500 mg momicine with mastercardIn fetal mice and monkeys antibiotics for acne for sale buy momicine 500 mg with visa, the drug accumulates for long intervals infection in blood purchase momicine 500 mg without a prescription, 5 months in mice treatment for dogs with flea allergies momicine 100mg with amex, within the melanin structures of the eyes and internal ears (3 virus contagious momicine 100 mg lowest price,4). However, in rats, only excessive doses have been teratogenic, producing skeletal and ocular defects (4). In pregnant mice, chloroquine alone was not teratogenic, however in combination with radiation, a significant enhance in cleft palates and tail anomalies was noticed. The Collaborative Perinatal Project monitored 50,282 motherchild pairs, 2 of whom had 1st trimester publicity to hydroxychloroquine (8). The fetus had been uncovered to the antimalarial agent, 200 mg twice daily, because the time of conception. Examination of the temporal bones, the embryonal precartilage, the anlages of the auditory ossicles, and the membranous labyrinth demonstrated a normal 14-week stage of growth, indicating an apparent lack of drug-induced ototoxicity in this fetus (9). A brief 1983 communication described using 200 mg/day of hydroxychloroquine in the course of the first 16 weeks of gestation for the therapy of maternal discoid lupus erythematosus (10). In 17 of the pregnancies, the drug was used at a dose of 200400 mg every day all through gestation. The outcomes of those instances included 2 miscarriages, 2 perinatal deaths, 1 infant with congenital coronary heart block (in a Ro-positive mother), and 12 regular newborns. In six different pregnancies, hydroxychloroquine was began after conception, three during the 1st trimester. In the remaining three cases, therapy was stopped after diagnosis of pregnancy, leading to a worsening of the disease and higher doses of prednisolone, and being pregnant termination in one because of severe renal lupus. The authors concluded that hydroxychloroquine was secure in being pregnant, and because of the chance of lupus flare, discontinuing therapy during pregnancy represented a larger danger to the fetus (5). These authors described nine pregnancies (plus seven from an earlier paper) in which hydroxychloroquine (200 mg/day) was used throughout gestation with out producing congenital malformations. In the current series of nine pregnancies, 5 newborns were delivered preterm and four at term. The use of hydroxychloroquine as an antimalarial, as an alternative of chloroquine, has been recommended because of the belief that hydroxychloroquine is less poisonous (4). However, few data can be found to substantiate this practice, either in phrases of congenital malformations or in optic or otic toxicity. From published reviews of fetal exposure to either chloroquine or hydroxychloroquine, one source cited an incidence of seven infants with congenital anomalies from 188 stay births-a rate of four. This worth is throughout the expected 3%6% incidence of congenital malformations in a nonexposed inhabitants. A 27-year-old lady was handled with hydroxychloroquine, 400 mg (310 mg base) every evening, for an exacerbation of lupus erythematosus (11). The authors estimated that the toddler was consuming, based on one thousand mL of milk, a every day dose of 1. Much lower milk concentrations of drug were obtained from a 28-year-old lady who was being treated with hydroxychloroquine, 200 mg twice daily, for rheumatoid arthritis (12). Treatment had been stopped for six months throughout pregnancy after which restarted 2 months later because of arthritis relapse. Because of the sluggish elimination fee and the potential for accumulation of a toxic quantity in the infant, breastfeeding throughout daily therapy with hydroxychloroquine must be undertaken cautiously (1113). The administration of once-weekly doses, similar to these used for malaria prophylaxis, would markedly scale back the quantity of drug obtainable to the nursing infant and, consequently, produce a much lower threat of accumulation and toxicity. The American Academy of Pediatrics classifies the drug as compatible with breastfeeding (14). Effects of antiinflammatory and immunosuppressive medication on being pregnant and fertility. The security of hydroxychloroquine in lupus pregnancy: experience in 27 pregnancies (abstract). Use earlier than this carries a small risk of female masculinization and hypospadias and/or ambiguous genitals in males. Because a quantity of points still exist, including long-term security and the best progesterone formulation. An enhance within the expected frequency of cardiovascular defects and hypospadias was noticed for each estrogens and progestogens (4, p. The congenital defects included spina bifida, anencephalus, hydrocephalus, tetralogy of Fallot, frequent truncus arteriosus, cataract, and ventricular septal defect. However, using diazepam in early pregnancy and the shortage of comparable stories make an affiliation uncertain. In 2000, Schardein (9) reviewed the risk of progestogens for female masculinization and male feminization after 1st trimester use. A 1985 examine described 2754 offspring born to mothers who had vaginal bleeding in the course of the 1st trimester (10). Of the entire group, 1608 of the newborns had been delivered from mothers treated during the 1st trimester with both oral medroxyprogesterone (2030 mg/day), 17-hydroxyprogesterone (500 mg/week by injection), or a mix of the 2. There have been no variations between the study and control groups in the total fee of malformations (120 vs. Another 1985 examine compared 988 infants exposed in utero to various progesterones with a matched cohort of 1976 unexposed controls (11). Hormone-exposed males demonstrated a development to have much less heterosexual experience and fewer masculine interests than controls (12). Congenital malformations among offspring exposed in utero to progestins, Olmsted County, Minnesota, 19361974. Suppression of threatened premature labor by administration of cortisol and 17-hydroxyprogesterone caproate: a comparability with ritodrine. Evaluation of the usage of proluton-depot (hydroxyprogesterone hexanoate) in early pregnancy. The effect of 17-hydroxyprogesterone caproate on being pregnant end result in an active-duty military inhabitants. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. Inefficacy of 17-hydroxyprogesterone caproate in the prevention of prematurity in twin being pregnant. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm delivery within the United States. Does progesterone therapy affect risk elements for recurrent preterm delivery? Progesterone for prevention of recurrent preterm delivery: impression of gestational age at previous delivery. Impact of the current randomized trials on using progesterone to stop preterm delivery: a 2005 follow-up survey. Increased recurrence of preterm delivery with early cessation of 17-alpha-hydroxyprogesterone caproate. Progestational agents to prevent preterm births: a metaanalysis of randomized controlled trials. Endocrine results of 17 alphahydroxyprogesterone caproate during early pregnancy: a double-blind medical trial. The effect of 17-hydroxyprogesterone caproate/oestradiol valerate on the development and consequence of early pregnancies following in vitro fertilization and embryo switch: a potential and randomized controlled trial.
Purchase 100mg momicineIt is indicated infection viral purchase 250mg momicine with mastercard, either alone or with different brokers antibiotic jaundice generic 500mg momicine mastercard, for the treatment of hypertension virus 09 generic momicine 500 mg mastercard. Plasma protein binding infection 2004 purchase momicine 500 mg with visa, primarily to albumin, is excessive (>99%) and the elimination half-life is about eleven hours (1). The molecular weight (about 607) and the elimination half-life recommend that the drug will cross to the embryofetus, even though the excessive plasma protein binding ought to limit the exposure. However, other agents on this class are known to cross the placenta and the same ought to be expected for azilsartan. The molecular weight (about 607) and the elimination half-life (about 11 hours) suggest that the drug will be excreted into breast milk, however the excessive plasma protein binding should limit the quantity excreted. It is in the macrolide anti-infective class that includes clarithromycin and erythromycin. Animal studies using rats and mice treated with day by day doses as much as maternal poisonous levels revealed no impairment of fertility or hurt to the fetus. No evidence of impaired fertility was found in rats given daily doses up to about zero. In 20 ladies scheduled for elective cesarean section, a single 1-g oral dose of azithromycin was given 6 (N = 2), 12 (N = 7), 24 (N = 5), 72 (N = 5), or 168 (N = 1) hours earlier than supply. The mean maternal concentrations at supply for the five teams have been 311, a hundred and forty four, sixty three, 60, and <10 ng/mL, respectively, whereas the corresponding imply twine serum levels had been 19, 26, 27, 19, and <10 ng/mL, respectively. Cerebrospinal fluid levels in the mothers (all had spinal anesthesia) were undetectable (<16 ng/mL) in every group. In an ex vivo experiment with time period human placentas using a single placental cotyledon model, the imply transplacental transfer of three macrolide antibiotics (azithromycin, erythromycin, and roxithromycin) had been 2. The percentages were calculated because the ratio between the steady-state level in fetal venous and maternal arterial sides (3). A number of reports (422) have described using azithromycin in human being pregnant. A 1994 abstract reported that sixteen pregnant sufferers with cervicitis attributable to Chlamydia had been treated with a single 1-g oral dose of the antibiotic in a comparison trial with erythromycin (4). No information got on gestational age at the time of treatment or on the pregnancy outcomes. In a second, related report, also comparing efficacy with erythromycin, 15 pregnant ladies with chlamydial cervicitis have been handled with a single 1-g oral dose (5). Three more modern stories have additionally documented the efficacy of azithromycin in the treatment of pregnant ladies with Chlamydia (68). Of the 5 stories, solely the last research (8) indicated the gestational age at treatment (about 24 weeks), but none offered info on fetal outcome. Two ladies with scrub typhus (tsutsugamushi disease) in the 2nd trimester have been treated efficiently with 3-day programs of azithromycin (10). A 1998 noninterventional observational cohort research described the outcomes of pregnancies in women who had been prescribed 1 of 34 newly marketed drugs by common practitioners in England (11). The outcomes of these pregnancies were 1 elective abortion and 10 regular, term babies (11). In a 2005 abstract, 145 pregnant ladies were uncovered to a new macrolide (38 azithromycin, 53 clarithromycin, and fifty four roxithromycin), of which 103 have been exposed within the 1st trimester (12). In a 2006 examine, comparisons have been made between three groups each containing 123 pregnancies: azithromycin (88 exposed in 1st trimester), nonteratogenic antibiotics, and nonteratogenic agents (13). The authors concluded that azithromycin was relatively protected during pregnancy (13). A retrospective cohort research utilizing knowledge from Tennessee Medicaid included 30,049 infants born in 19852000 was printed in 2009 (14). Infants with fetal exposures in the 1st trimester to four antibiotics beneficial for potential bioterrorism attacks (azithromycin, amoxicillin, ciprofloxacin, and doxycycline) were compared with infants with no fetal exposure to any antibiotic. A 2009 case report described the use of azithromycin (500 mg/day for six days) for the treatment of Q fever in the 9th week of pregnancy (15). A second research using this same group of patients found, in addition to the decrease rates of an infection, decreased rates of neonatal demise, low birth weight, and preterm supply (17). However, in a 3rd study involving the same Uganda women as above, children of 94 women with Trichomonas vaginalis who had been treated during being pregnant with the three-drug combination had elevated low delivery weight (<2500 g) and preterm delivery fee compared with untreated controls (18). The authors concluded that treatment of the condition throughout pregnancy was dangerous and that it was more than likely as a result of metronidazole. A prospective multicenter examine printed in 2008 compared pregnant ladies uncovered to a brand new macrolide (azithromycin, clarithromycin, or roxithromycin) with two comparison teams (19). A potential, multicenter observational 2012 study was performed by teratogen info services in Italy, Israel, Czech Republic, the Netherlands, and Germany (20). The research group was compared with 773 ladies uncovered to nonteratogens in the 1st trimester. Two reviews, one an summary, have examined the potential of azithromycin in the prevention of premature birth (21,22). The results supplied no evidence that using the antibiotic might stop preterm birth (22). In a 2003 Danish examine, 188 women acquired a macrolide (see Breastfeeding Summary) within 30 days of start and none of their infants had childish hypertrophic pyloric stenosis (23). She was discharged from the hospital on a 5-day course of azithromycin, 500 mg every day, but only took three doses because she wanted to resume breastfeeding that had been stopped during azithromycin remedy. The patient continued pumping her breasts during this time to maintain milk circulate and resumed breastfeeding 24 hours after the third dose of the antibiotic. Drug doses and approximate time from the primary dose had been 1 g (0 hours), 500 mg (59 hours), 500 mg (83 hours), and 500 mg (107 hours). A 2003 examine investigated the association between maternal use of macrolides and infantile hypertrophic pyloric stenosis (23). The Danish population-based cohort study comprised 1166 ladies who had been a prescribed macrolide (azithromycin, clarithromycin, erythromycin, spiramycin, or roxithromycin) from start to ninety days postnatally in contrast with as a lot as 41,778 controls. Investigators from Israel examined the attainable affiliation between macrolide (azithromycin, clarithromycin, erythromycin, or roxithromycin) exposure in milk and infantile hypertrophic pyloric stenosis in a 2009 research (25). They compared fifty five infants exposed to a macrolide antibiotic with 36 infants uncovered to amoxicillin. The transplacental switch of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. Comparison of azithromycin and erythromycin for Chlamydia cervicitis in pregnancy (abstract). Azithromycin and erythromycin in the therapy of cervical chlamydial an infection throughout pregnancy. A randomized trial of erythromycin and azithromycin for the remedy of chlamydia an infection in pregnancy (abstract). A randomized medical trial of a single dose, of azithromycin in the therapy of chlamydia among pregnant women (abstract). Efficacy of azithromycin in reducing lower genital ureaplasma colonization in girls at risk for preterm supply (abstract). Pregnancy end result after gestational publicity to the new macrolides: a prospective controlled cohort research (abstract).
Momicine 500 mg genericThe latter dose is suspended in a mixture of propellants (dichlorodifluoromethane antibiotics you can give dogs purchase momicine 250 mg without a prescription, trichloromonofluoromethane antibiotics klacid xl purchase 100 mg momicine with visa, and dichlorotetrafluoroethane) and sorbitan trioleate antibiotic wound infection momicine 500mg otc. Approximately 34% of the delivered dose of Pulmicort and 21% of Rhinocort are absorbed systemically (2 antibiotic iv therapy generic momicine 100mg free shipping,3). The comparatively low molecular weight (about 431 for the butyraldehyde formulation) and high lipid solubility recommend substantial placental transfer. The actual amount of lively budesonide reaching the fetus, nonetheless, may be small due to the low systemic bioavailability after inhaled or nasal administration (see above). Data from the Swedish Medical Birth Registry involving 2014 infants whose mothers had inhaled budesonide for asthma throughout early being pregnant were reported in 1999 (5). Drug exposures had been recognized prospectively, earlier than the being pregnant outcomes were known. Among the mothers of the 2014 infants, 1675 additionally used 2-adrenergic agonists, 16 used other inhaled corticosteroids in addition to budesonide, and 316 used no different antiasthmatic drug. Major structural defects have been noticed in forty one of the infants, whereas 35 infants had minor and/or variable circumstances. Among the major and minor defects, 18 (expected 1718) concerned the heart, including 2 premature infants with patent ductus arteriosus (both categorized as minor defects). There had been now 2968 mothers who had used inhaled budesonide in early being pregnant and gave birth to infants with regular gestational age, start weight, and length. Twenty-one pregnancies in which the woman acquired an energetic drug were reported to the manufacturer during scientific trials of Pulmicort Turbuhaler (A. Two of the pregnancies were terminated voluntarily, 1 girl had a miscarriage, 3 had unknown outcomes, thirteen delivered regular infants, and 2 delivered infants with congenital malformations. In one case, the 27-year-old mother was receiving budesonide 1600 mcg/day at conception. She was also taking prednisone 10 mg/day and a mix oral contraceptive (levonorgestrel + ethinyl estradiol). In the second case, a 41-year-old mom used budesonide inhaler for 22 days (dosage unknown) earlier than discontinuing it because of her being pregnant. Seven months later, she delivered a feminine toddler with double-sided maxillary clefts, double digits of the left hand, and chronic fetal circulation. The toddler outcomes of five asthmatic girls who used budesonide during pregnancy had been described in retrospective postmarketing reviews to the producer (A. The first case involved a 36-year-old woman, treated with an unknown dose of budesonide combined with a 2-adrenergic agonist (albuterol), who delivered an anencephalic infant. In the second case, a 32-year-old woman administering budesonide 1600 mcg/day gave birth to a healthy 2. A 33-year-old lady was handled with 4 antiasthmatic agents during gestation, including budesonide 1600 mcg/day, an oral corticosteroid (name not specified), a 2adrenergic agonist (not specified), and theophylline. She gave delivery to a female toddler with agenesis of the left foot who was in any other case healthy. Another 33year-old girl being handled with budesonide 1600 mcg/day, phenobarbital, and terbutaline (a 2-adrenergic agonist) delivered an toddler with cleft palate, an unspecified cardiac defect, and hydrocephalus. A 2003 casecontrol examine, using information from three Swedish health registers, was conducted to establish drug use in early being pregnant that was related to cardiac defects (7). Cases of cardiovascular defects without recognized chromosome anomalies (N = 5015) had been compared with controls consisting of all infants born in Sweden (19952001) (N = 577,730). The analysis included 5 agents: beclomethasone, budesonide, flunisolide, fluticasone, and triamcinolone. Although most synthetic corticosteroids are partially metabolized by placental enzymes, budesonide was not (10). However, the systemic bioavailability of budesonide following inhalation therapy is comparatively low (see section above), thus, the actual quantity in milk additionally may be low. In a 2007 examine, milk and plasma budesonide concentrations have been measured in eight mothers receiving maintenance therapy with Pulmicort Turbuhaler (200 or four hundred mcg twice daily) (11). The common plasma concentrations in nursing infants was estimated to be 1/600th of the maternal concentration (all toddler levels have been less than the restrict of quantification), assuming 100% bioavailability within the infants. The authors concluded that their knowledge supported continued use of inhaled budesonide throughout breastfeeding (11). Normal pregnancy outcomes in a population-based study together with 2968 pregnant women uncovered to budesonide. In rabbits, that are extra delicate to the results of bumetanide than other check species, doses three. In a surveillance study of Michigan Medicaid recipients involving 229,one hundred and one completed pregnancies carried out between 1985 and 1992, 44 newborns had been exposed to bumetanide through the 1st trimester (F. Bumetanide probably is compatible with breastfeeding however, in general, diuretics ought to be used cautiously throughout nursing as a end result of they may suppress lactation. One pregnancy case has been situated by which the drug was given as a narcotic substitute for heroin dependency. Neonatal withdrawal was noticed, but the symptoms have been lower than that anticipated with methadone. Animal research have demonstrated dose-related maternal, embryo, and fetal toxicity and dose-related behavioral modifications in offspring, but no congenital malformations. Although the shortage of congenital anomalies is reassuring, the behavioral changes in animals mixed with the absence of printed early human being pregnant experience prevent an evaluation of the chance that this drug presents to the embryo or fetus. Buprenorphine might have a job as substitution remedy for maternal heroin habit, however extra reviews are wanted to outline its being pregnant security profile before this use can be really helpful. Although a sublingual formulation is available in other countries, solely parenteral buprenorphine has been approved for general use in the United States. Buprenorphine is currently beneath investigation as a substitute for methadone maintenance therapy of narcotic dependence (13). Of interest, however, abuse of buprenorphine, typically concurrently with opiates, has been reported outside of the United States (4,5). One of those latter citations reported frequent abuse of buprenorphine by pregnant girls however provided no consequence information (4). Shepard (7) cited a research by which fetal growth restriction, but not congenital defects, was observed in rats exposed to maternal doses of >0. Rat fetuses exposed to 5 mg/kg/day in the final week of being pregnant had a reduced survival rate after supply (7). Minimal results have been noticed on the endogenous opioid system, nevertheless, as decided by a comparability of the brain enkephalin levels of buprenorphine-exposed pups to those in methadoneexposed pups (9). In a research involving pregnant rats, buprenorphine was administered by a steady infusion in doses of 0. At these doses, no evidence of great maternal toxicity was observed, nor were there any important effects on the offspring by means of morbidity and mortality, start weight, and postnatal development (up to 60 days) (10). In a second publication by these researchers, utilizing the identical drug-administration approach and animal type described above, no disruption in the restactivity cycle was observed within the exposed offspring at 22 and 30 days of age (11). The long-term results on sexual differentiation in rats uncovered in utero to maternal injections of buprenorphine, zero. Compared with controls and the lower-dose group, spontaneous parental conduct (at 2328 days of age) and the expected sex difference within the consumption of a zero.
Buy 100 mg momicine visaReproduction research have been carried out with cefditoren pivosil in rats and rabbits treatment for dogs with dementia order momicine 100mg online. However antibiotics for dogs australia cheap momicine 500mg fast delivery, in rabbits bacteria gif 100mg momicine with visa, the highest dose triggered severe maternal toxicity leading to fetal toxicity and abortions infection mrsa pictures and symptoms discount 100 mg momicine amex. Small quantities of antibiotic have been present in breast milk for all cephalosporins which have been studied, so the presence of cefditoren in milk ought to be anticipated. However, three potential issues exist for the nursing toddler exposed to cefditoren in milk: modification of bowel flora, direct effects on the toddler, and interference with the interpretation of culture outcomes if a fever workup is required. The American Academy of Pediatrics classifies other cephalosporins as suitable with breastfeeding (for instance, see Cefadroxil and Cefazolin). Consistent with the molecular weight (about 481 for the free base), cefepime crosses the human placenta. The transplacental passage rate calculated as a proportion of fetal venous focus to maternal blood focus was about 23%, whereas the transfetal passage rate calculated as a percentage of fetal arterial drug concentration to fetal venous concentration was about 79% (2). The producer reviews that a nursing infant consuming about one thousand mL of human milk per day would acquired about zero. In spite of this low dose, three potential problems exist for the nursing toddler exposed to cefepime in milk: modification of bowel flora, direct effects on the toddler, and interference with the interpretation of tradition results if a fever workup is required. Although not particularly listing cefepime, the American Academy of Pediatrics classifies different cephalosporin antibiotics as appropriate with breastfeeding (for instance, see Cefadroxil and Cefazolin). Reproduction studies discovered no proof in rats of impaired fertility or reproductive efficiency at doses as a lot as 125 occasions the grownup therapeutic dose or, in mice and rats, of teratogenicity at doses as much as four hundred occasions the human dose (1). Consistent with the molecular weight (about 453), cefixime crosses the human placenta. A 2010 examine measured cefixime amniotic fluid concentrations in six women given a 400 mg oral dose about 2 hours earlier than amniocentesis (2). A 1998 noninterventional observational cohort examine described the outcomes of pregnancies in girls who had been prescribed one or more of 34 newly marketed medication by general practitioners in England (3). The outcomes of those pregnancies included two spontaneous abortions, one elective abortion, seven regular newborns (one premature), and one unknown consequence (3). A 2003 research evaluated 95 pregnant girls with gonorrhea who had been treated with either cefixime (N = 52) or ceftriaxone (N = 43) (4). Hyperbilirubinemia occurred more often in infants from moms handled with ceftriaxone, but the difference was not vital (4). A second examine utilizing this similar group of patients found, in addition to the lower charges of an infection, decreased charges of neonatal dying, low start weight, and preterm delivery (6). However, in a 3rd study involving the identical Uganda ladies as above, youngsters of ninety four women with Trichomonas vaginalis who had been handled during pregnancy with the 3-drug combination had increased low delivery weight (<2500 g) and preterm start rate compared with untreated controls (7). Low concentrations of different cephalosporins have been measured, however, and the presence of cefixime in milk ought to be expected. Three potential issues exist for the nursing toddler uncovered to cefixime in milk: modification of bowel flora, direct effects on the infant, and interference with the interpretation of tradition results if a fever workup is required. A randomized trial that in contrast oral cefixime and intramuscular ceftriaxone for the therapy of gonorrhea in being pregnant. Randomized trial of presumptive sexually transmitted illness remedy during being pregnant in Rakai, Uganda. Treatment of Trichomonas in being pregnant and opposed outcomes of being pregnant: a subanalysis of a randomized trial in Rakai, Uganda. Reproduction research in mice and rats have discovered no evidence of impaired fertility or fetal harm at doses as a lot as 20 times the human dose (1). Five ladies with chorioamnionitis and in labor acquired cefotaxime (dose not specified) (3). The American Academy of Pediatrics classifies cefotaxime as suitable with breastfeeding (5). Antibiotic concentration in maternal blood, twine blood and placental tissue in ladies with chorioamnionitis. Reproduction research in rats and monkeys discovered no evidence of impaired fertility or fetal harm at doses up to 20 instances the human dose (1). A 1985 study measured the placental passage of the drug when administered just previous to cesarean section (2). Cord blood concentrations progressively increased depending on the length of time after a mom obtained a dose and had been highest (12. Similarly, a progressive increase in amniotic fluid concentrations was observed with values of 5. The increases within the level of antibiotic in the amniotic fluid paralleled those in the cord blood. No accumulation within the milk was noticed, as evidenced by a steady milk:plasma ratio. Even though the quantities of antibiotic are very small, and no reports of antagonistic effects in a nursing infant have been located, three potential problems exist for the infant exposed to cefotetan in milk: modification of bowel flora, direct results on the infant, and interference with the interpretation of tradition results if a fever workup is required. Although not specifically itemizing cefotetan, the American Academy of Pediatrics classifies different cephalosporin antibiotics as compatible with breastfeeding (for example, see Cefadroxil and Cefazolin). The penetration of intramuscular cefotetan disodium into human extra-vascular fluid and maternal milk secretion. Amniotic fluid concentrations peaked at 23 hours in the 315 mcg/mL range (7,8,10,11,14). Up to 2 mcg/mL has been detected in the milk of girls receiving therapeutic doses (J. Following prophylactic administration of 24 g of cefoxitin to 18 ladies during and following cesarean part, milk samples had been collected a imply 25 hours (range 956 hours) after the last dose of antibiotic (15). Only one pattern, collected 19 hours after the final dose, contained measurable concentrations of cefoxitin (0. Although these ranges are low, three potential problems exist for the nursing toddler: modification of bowel flora, direct effects on the toddler, and interference with the interpretation of tradition results if a fever workup is required. The American Academy of Pediatrics classifies cefoxitin as suitable with breastfeeding (16). Bergone-Berezin B, Kafe H, Berthelot G, Morel O, Benard Y Pharmacokinetic research of cefoxitin in. In: Current Chemotherapy: Proceedings of the 10th International Congress of Chemotherapy, Zurich, Switzerland, September 1823, 1977. Berthelot G, Bergogne-Berezin B, Morel O, Kafe H, Benard Y Cefoxitin: Pharmacokinetic Study in. Paper Presented at 10th International Congress of Chemotherapy, Zurich, Switzerland, September 1823, 1977. Laboratory and medical studies on cefoxitin in the area of obstetrics and gynecology.
Buy momicine 100 mg with visaSamples of maternal and fetal blood had been obtained earlier than supply at 30 antibiotics for acne bad 100mg momicine amex, ninety best antibiotics for acne uk generic momicine 250 mg without a prescription, and 120 210 minutes after the dose in 7 virus fever buy 100mg momicine amex, eight infection list purchase 100mg momicine overnight delivery, and 7 girls, respectively. Mean maternal blood concentrations of fosfomycin on the three time intervals were 14. Another report found that the drug crossed the placenta, resulting in detectable concentrations in amniotic fluid and twine blood in three girls (3). The mean most maternal serum focus of fosfomycin, after a single 3-g oral dose of fosfomycin tromethamine beneath fasting conditions, was 26. As ought to be expected because of the conventional physiologic changes that happen during gestation, pregnant ladies will have decrease peak ranges. Although acceptable precautions had been taken to exclude and stop pregnancies during clinical trials, three girls conceived shortly after enrolling and all acquired a single 3-g oral dose of fosfomycin (H. The dose was apparently consumed about three days earlier than conception in a single case, 8 days after the final menstrual period (probably earlier than conception) in a second, and 14 days after the last menstrual interval (assumed to be across the time of conception) in a third. The first lady was lost to follow-up and the other two delivered wholesome male newborns who have been growing usually at three years of age. About 5 days later, ultrasound demonstrated no fetal heartbeat and an induced abortion was performed. The explanation for death was thought to be caused by progressive a number of placental infarctions and fetal hypotrophy. Several revealed reviews have studied the efficacy and security of oral fosfomycin during being pregnant (415). The drug has been utilized in all trimesters of pregnancy with out obvious hurt to the fetus or newborn. A 1998 noninterventional observational cohort research described the outcomes of pregnancies in ladies who had been prescribed 1 of 34 newly marketed medicine by general practitioners in England (16). Of 1067 exposed pregnancies, fosfomycin was taken through the 1st trimester in two, both concluding with regular, full-term infants. However, consistent with the molecular weight (about 259), the drug is excreted into colostrum and breast milk (3). Urinary tract infections in being pregnant: Monuril single-dose therapy versus traditional remedy. Fosfomycin Trometamol Single Dose versus Pipemidic Acid 7 Days within the Treatment of Bacteriuria in Pregnancy. Treatment of bacteriuria in being pregnant with single dose fosfomycin trometamol: a evaluation. Monuril Effectiveness and Tolerability within the Treatment and Prevention of Urinary Tract Infections. Efficacy and Safety of Fosfomycin Trometamol in the Treatment of Bacteriuria in Pregnancy. Fosfomycin trometamol versus pipemidic acid in the therapy of bacteriuria in being pregnant. Marone P, Concia E, Catinella M, Andreoni M, Guaschino S, Marino L, Grossi F, Cellani F. Fosfomycin trometamol in the treatment of urinary tract infections throughout being pregnant. In: 3rd International Congress, Infections in Obstetrics and Gynecology, Pavia, Italy, 1988. Fosfomycin trometamol single-dose in the remedy of uncomplicated urinary tract infections in cardiac pregnant or nonpregnant girls. Two comparability teams, these taking different antihypertensives (N = 202) and people taking no antihypertensives (N = 29,096), were shaped. The reason for the defects and different toxicity is thought to be associated to fetal hypotension and decreased renal blood flow. Since the primary technique of removal of the drug is renal, the impairment of this method in the newborn prevents elimination of the drug, resulting in prolonged hypotension. If oligohydramnios happens, stopping fosinopril may resolve the problem however may not enhance toddler outcome due to irreversible fetal harm (6). The components that sometimes coexist with hypertension in pregnancy included diabetes, superior maternal age, and weight problems. The manufacturer states that the drug can be detected in milk after a daily dose of 20 mg given for 3 days (1). Although the results of this exposure on a nursing toddler are unknown, the American Academy of Pediatrics classifies two other similar agents (see Captopril and Enalapril) as compatible with breastfeeding. Risks of angiotensin-converting enzyme inhibition throughout being pregnant: experimental and medical proof, potential mechanisms, and recommendations for use. Although the animal knowledge suggest low threat, human data are required for a whole assessment of the embryofetal risk. As with propofol, use of the prodrug close to birth might trigger transient depression of the new child (see Propofol). It is indicated for monitored anesthesia care sedation in adult patients undergoing diagnostic or therapeutic procedures. Both fospropofol and its active metabolite propofol are extremely plasma protein certain (about 98%) primarily to albumin (1). In rats, the best doses triggered vital maternal toxicity, whereas in rabbits all doses brought on important maternal toxicity. However, in a 3rd assay, fospropofol was mutagenic in the presence of metabolic activation but the discovering was thought to have been an artifact of the culture conditions (1). The molecular weight (about 332 for the disodium salt form) is low enough, but the extensive plasma protein binding and comparatively quick elimination half-life recommend that switch will be limited. However, the energetic metabolite propofol does cross, with an umbilical vein:maternal vein ratio of zero. The molecular weight (about 332 for the disodium salt form) is low enough for excretion, but the extensive plasma protein binding and comparatively brief elimination half-life ought to limit the quantity in milk. Small quantities of the energetic metabolite propofol are excreted into colostrum and milk following use of the agent for the induction and maintenance of maternal anesthesia during cesarean supply (see Propofol). The drug is carefully related to almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan. Dose-related increases had been found in the incidences of dilated ureters, unilateral and bilateral pelvic cavitation, hydronephrosis, and hydroureters. The renal effects have been thought to be in maintaining with a slight delay in fetal maturation. Skeletal variations (incomplete ossification of the sternebrae, cranium, and nasal bones) had been noticed at all doses. The molecular weight of the parent compound (about 243 for the free base) is low sufficient that passage to the fetus ought to be expected.
Momicine: 500 mg, 250 mg, 100 mg
Order 250mg momicine mastercardVenlafaxine was not detected in toddler plasma virus 59 best momicine 100mg, however the median toddler metabolite plasma concentration was a hundred mcg/L (range 23225 mcg/L) infection 3 months after wisdom teeth extraction purchase momicine 100 mg otc. Venlafaxine was detected in the plasma of one infant (5 mcg/L) antibiotic resistance buy momicine 100mg without a prescription, whereas desvenlafaxine was detected in four infants (range 338 mcg/L) how quickly do antibiotics for uti work cheap 250 mg momicine otc. The highest venlafaxine and desvenlafaxine concentrations in milk occurred 8 hours after maternal ingestion. A study of a woman taking desvenlafaxine 250 mg/day and amisulpride (an atypical antipsychotic not available in the United States) 100 mg/day for despair and nursing her 5-month-old toddler was reported in 2010 (6). The absolute (theoretical) toddler doses of the two drugs were 294 and 183 mcg/kg/day, respectively. The infant was reaching anticipated developmental progress for age and no antagonistic effects had been famous (6). In a 2011 study of 10 women taking desvenlafaxine (50150 mg/day) and their nursing infants (mean age 4. Maternal plasma concentrations of desvenlafaxine and venlafaxine decide the amount of drug excreted into milk. In this regard, a 2009 research appears to have important implications for choosing which agent to use in a lactating girl (8). Additional studies, especially long-term follow-up of uncovered infants, are warranted. Distribution of venlafaxine and its O-desmethyl metabolite in human milk and their results in breastfed infants. Comparison of the pharmacokinetics of venlafaxine extended release and desvenlafaxine in in depth and poor cytochrome P450 2D6 metabolizers. However, depending on the indication, the benefit of therapy might outweigh the chance. In a casecontrol examine, the California Birth Defects Monitoring Program evaluated the affiliation between selected congenital anomalies and the usage of corticosteroids 1 month before to three months after conception (periconceptional period) (1). Although this therapy is supported by many clinicians, its use is still controversial since the useful effects of steroids are greatest in singleton pregnancies with feminine fetuses (1619). A current report, nonetheless, found no distinction within the incidence of maternal complications between handled and nontreated sufferers (23). Leukocytosis has been observed in infants exposed antenatally to dexamethasone (26,27). The use of corticosteroids, including dexamethasone, for the treatment of asthma throughout pregnancy has not been associated to a considerably elevated danger of maternal or fetal complications (28). An earlier study additionally recorded a shortening of gestation with chronic corticosteroid use (29). In Rh-sensitized women, the use of dexamethasone may have prevented intrauterine fetal deterioration and the need for fetal transfusion (30). Five girls, within the 2nd and 3rd trimesters, were treated with 24 mg of the steroid weekly for 27 weeks resulting, in each case, in a stay new child. Dexamethasone, 4 mg/day for 15 days, was administered to a woman late within the 3rd trimester for the remedy of autoimmune thrombocytopenic purpura (31). Therapy was given in an unsuccessful try to stop fetal/neonatal thrombocytopenia as a result of the placental transfer of antiplatelet antibody. Platelet counts within the newborn were 38,00049,000/mm3, however the infant made an uneventful restoration. The use of dexamethasone for the pharmacologic suppression of the fetal adrenal gland has been described in two girls with 21-hydroxylase deficiency (32,33). This deficiency results in the overproduction of adrenal androgens and the virilization of feminine fetuses. Dexamethasone, in divided doses of 1 mg/day, was administered from early within the 1st trimester (5th week and 10th week) to time period. Although human research have often shown a profit, the usage of corticosteroids in animals has been related to several toxic effects (34,35): Reduced fetal head circumference Reduced fetal adrenal weight Increased fetal liver weight Reduced fetal thymus weight Reduced placental weight Fortunately, none of these results has been observed in human investigations. Long-term follow-up evaluations of kids exposed in utero to dexamethasone have shown no opposed effects from this exposure (36,37). Changes in amniotic fluid lecithinsphingomyelin ratio following maternal dexamethasone administration. Human amniotic fluid lecithin/sphingomyelin ratio adjustments with estrogen or glucocorticoid treatment. Prevention of the respiratory distress syndrome in untimely infants by antepartum glucocorticoid therapy. The impact of glucocorticoids on the maturation of premature lung membranes: stopping the respiratory distress syndrome by glucocorticoids. Dexamethasone for prevention of respiratory, misery syndrome: multiple perinatal factors. Maternal administration of dexamethasone in severe pregnancy-induced hypertension. The argument for prenatal administration of dexamethasone to forestall respiratory misery syndrome. Effects of antenatal dexamethasone administration in the toddler: long-term follow-up. One research recorded 14 exposures within the 1st trimester without evidence for an affiliation with malformations (1). In a surveillance research of Michigan Medicaid recipients involving 229,101 completed pregnancies carried out between 1985 and 1992, 1080 newborns had been exposed to dexchlorpheniramine in the course of the 1st trimester (F. Specific information had been out there for six defect classes, together with (observed/expected) 10/11 cardiovascular defects, 2/2 oral clefts, 0/0. As with all drug therapy, avoidance of dexlansoprazole during being pregnant, particularly in the course of the 1st trimester, is the safest course. If dexlansoprazole is required or if inadvertent exposure does happen early in gestation, the recognized threat to the embryofetus for congenital defects seems to be low. It is in the same drug class as esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole. Plasma protein binding is about 96%99% and the elimination half-life is about 12 hours (1). At oral doses about 9 times the maximum really helpful human dose, no evidence of fetal harm was noticed (1). The molecular weight (about 369) and the elimination half-life suggest that the drug will cross to the embryofetus, but the excessive plasma protein binding may limit the exposure. A population-based, observational, cohort examine shaped by linking data from three Swedish national healthcare registers over a 10-year period (19952004) was reported in 2009 (3). The authors proposed three potential mechanisms for his or her findings: (a) publicity to increased quantities of allergens might trigger sensitization to digestion of labile antigens within the fetus; (b) maternal Th2 cytokine pattern may promote an allergy-prone phenotype within the fetus; and (c) maternal allergen-specific immunoglobulin E might cross the placenta and sensitize fetal immune cells to meals and airborne allergens. The molecular weight (about 369) and the elimination half-life (about 12 hours) suggest that the drug will be excreted into breast milk, but the excessive plasma protein binding (about 96%99%) might limit the amount excreted. Because of the carcinogenicity noticed in animals with lansoprazole, and the potential for suppression of gastric acid secretion within the nursing toddler, using dexlansoprazole during lactation is finest avoided.
References - Cutress ML, Stewart GD, Wells-Cole S, et al: Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience, BJU Int 110:1608-1617, 2012.
- Sobel M, Bohne WH, O'Brien SJ. Peroneal tendon subluxation in a case of anomalous peroneus brevis muscle. Acta Orthop Scand. 1992;63(6):682- 684.
- Coughlin M, Mann R, Saltzman C. Imaging of the foot and ankle. In: Surgery of the Foot and Ankle 8th ed. Philadelphia, PA: Elsevier; 2007:71- 131.
- Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA 2007;298:874-879.
- Lauren P: The two histological main types of gastric carcinoma: Diffuse and so-called intestinal type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31, 1965.
- Le Prise E, Etienne PL, Meunier B, et al: A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 73:1779, 1994.
|